Chapter: Plant Biochemistry: Nitrate assimilation is essential for the synthesis of organic matter
The end product of nitrate assimilation is a whole spectrum of amino acids
The end product of nitrate assimilation is a whole spectrum of amino acids
The carbohydrates formed as the product of CO2 assimilation are transported from the leaves via the sieve tubes to various parts of the plants. The transport forms of the carbohydrates are sucrose, sugar alcohols (e.g., sorbitol), or raffinoses, depending on the spe-cies. There are no such special transport forms for the products of nitrate assimilation. All amino acids present in the mesophyll cells are exported via the sieve tubes. Therefore the sum of amino acids can be regarded as the final product of nitrate assimilation. Synthesis of these amino acids takes place mainly in the chloroplasts. The pattern of the amino acids synthe-sized varies largely, depending on the species and the metabolic conditions. In most cases glutamate and glutamine represent the major portion of the synthesized amino acids. Glutamate is exported from the chloroplasts in exchange for malate and glutamine in exchange with glutamate (Fig. 10.6). Also, serine and glycine, which are synthesized as intermediate products in the photorespiratory cycle, represent a considerable portion of the total amino acids present in the mesophyll cells. Large amounts of alanine are often formed in C4 plants.
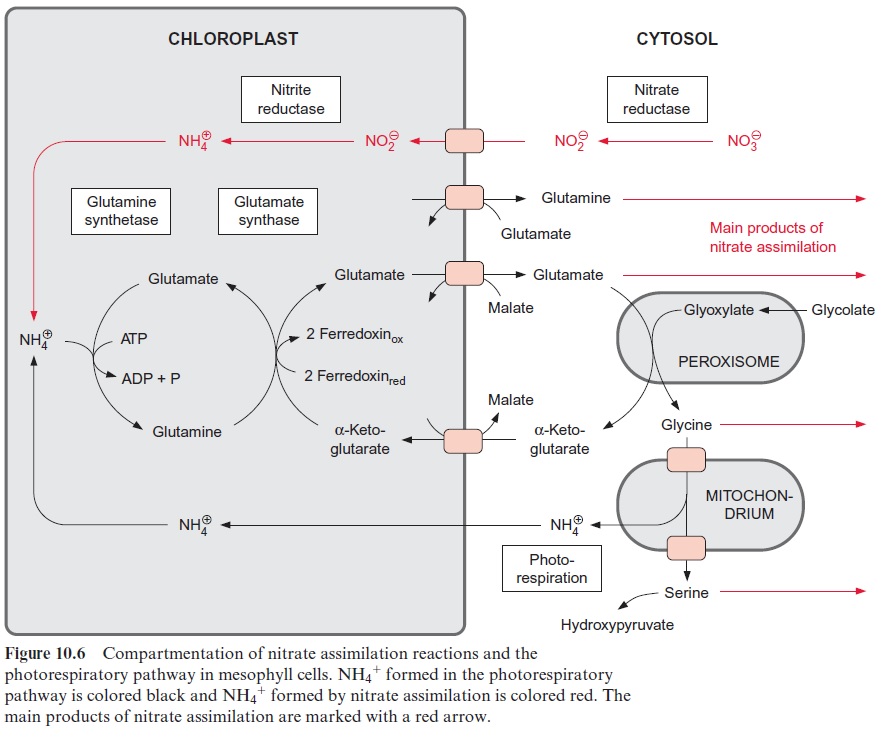
CO2 assimilation provides the carbon skeletons to synthesize the end products of nitrate assimilation
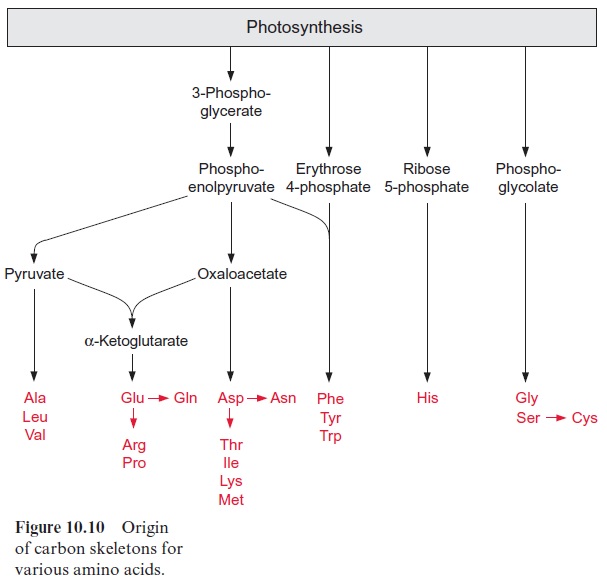
CO2 assimilation provides the carbon skeletons required for the synthesis of the various amino acids. Figure 10.10 gives an overview of the origin of the carbon skeletons of individual amino acids.
3-Phosphoglycerate is the most important carbon precursor for the syn-thesis of amino acids. It is generated in the Calvin cycle and exported from the chloroplasts to the cytosol by the triose phosphate-phosphate transloca-tor in exchange for phosphate (Fig. 10.11). 3-Phosphoglycerate is converted in the cytosol by phosphoglycerate mutase and enolase to phosphoenolpyru-vate (PEP). From PEP two pathways branch off, the reaction via pyru-vate kinase leading to pyruvate, and via PEP carboxylase to oxaloacetate.
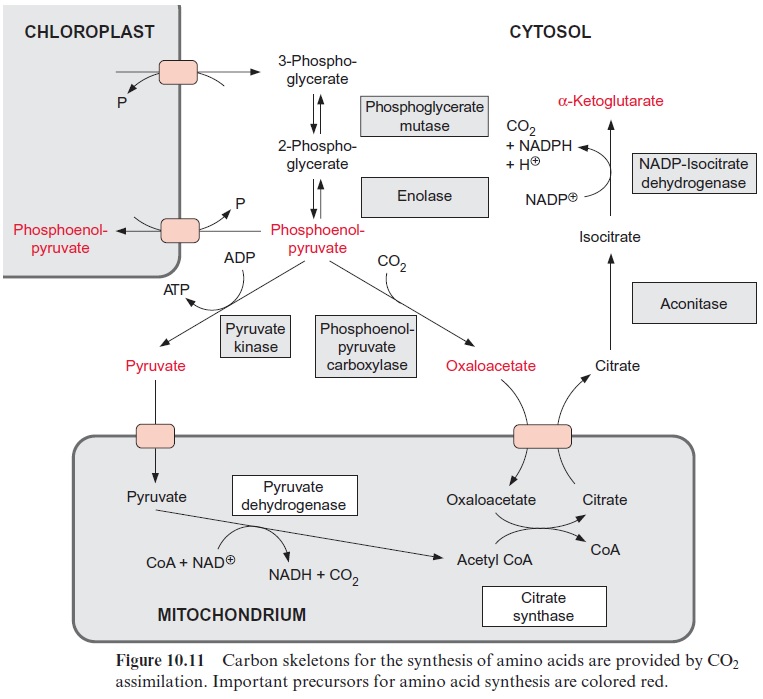
Moreover, PEP together with erythrose 4-phosphate is the precursor for the synthesis of aromatic amino acids via the shikimate pathway. Since the shikimate pathway is located in the chloro-plasts, the PEP required is transported via a specific PEP-phosphate trans-locator into the chloroplasts.
The PEP carboxylase reaction has already been discussed in conjunc-tion with the metabolism of stomatal cells and C4 and CAM metabolism. Oxaloacetate formed by PEP carboxy-lase has two functions in nitrate assimilation:
1. It is converted by transamination to aspartate, which is the precursor for the synthesis of five other amino acids (asparagine, threonine, iso-leucine, lysine, and methionine).
2. Together with pyruvate it is the precursor for the formation of -ketogl-utarate, which is converted by transamination to glutamate, being the precursor of three other amino acids (glutamine, arginine, and proline).
Glycolate synthesized by photorespiration is the precursor for the for-mation of glycine and serine (see Fig. 7.1), and from the latter cysteine is formed . In non-green cells, serine and glycine can also be syn-thesized from 3-phosphoglycerate. Details of this are not discussed here. Ribose 5-phosphate is the precursor for the synthesis of histidine. This pathway has not yet been fully resolved in plants.
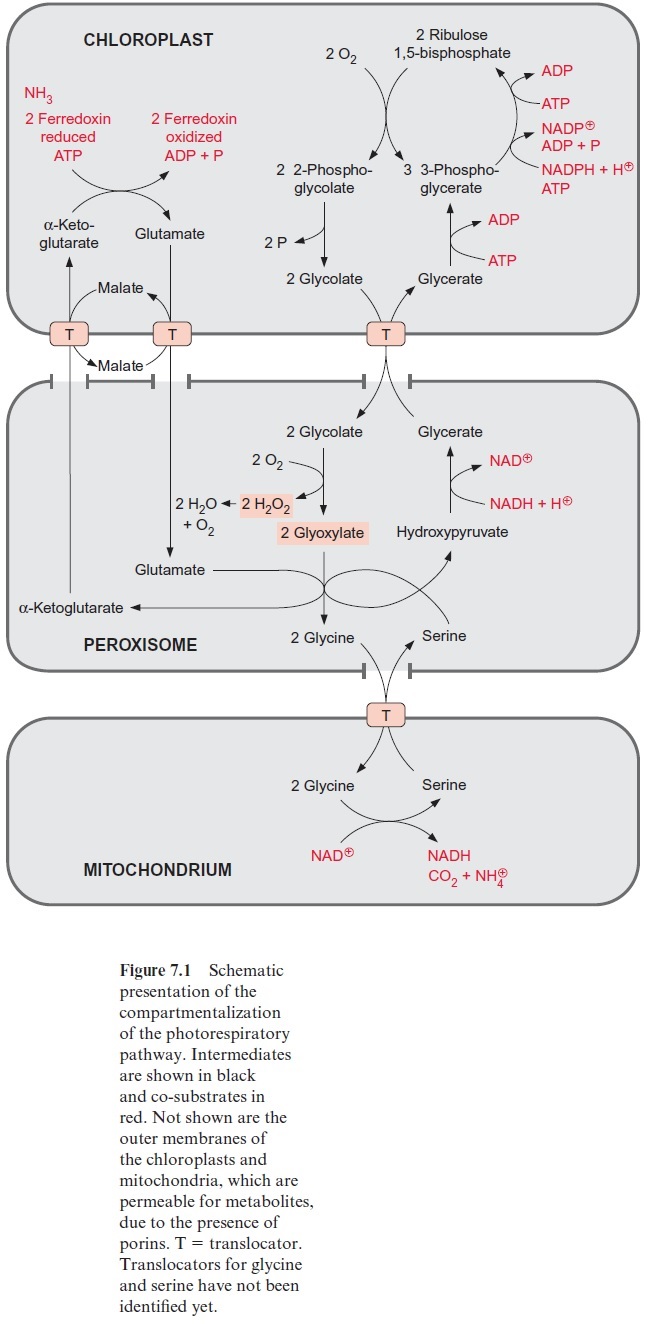
The synthesis of glutamate requires the participation of mitochondrial metabolism
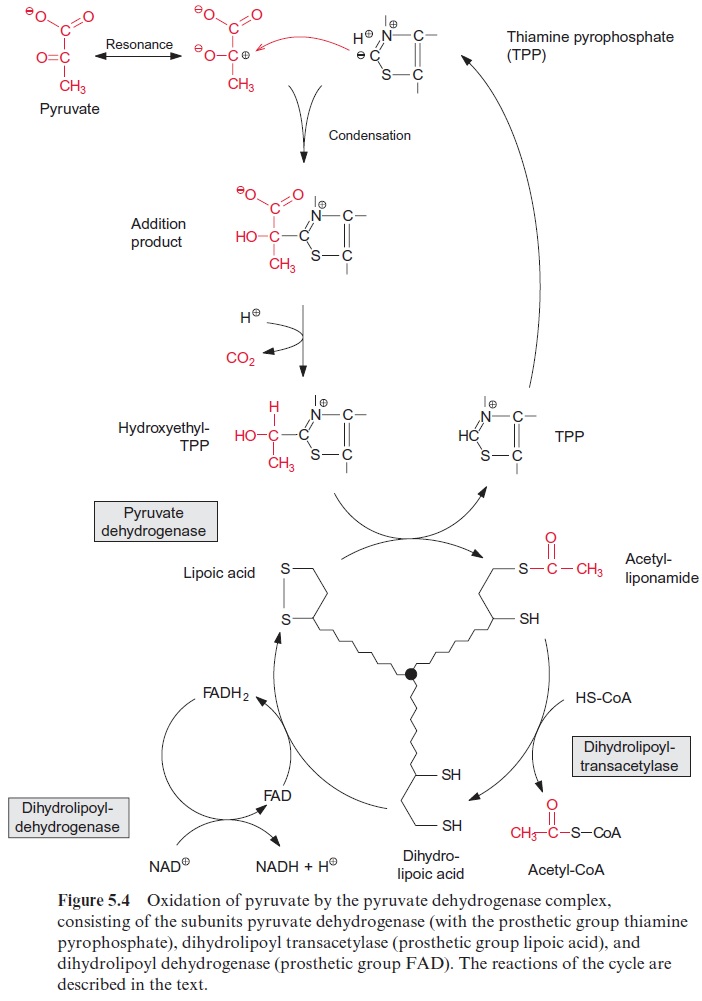
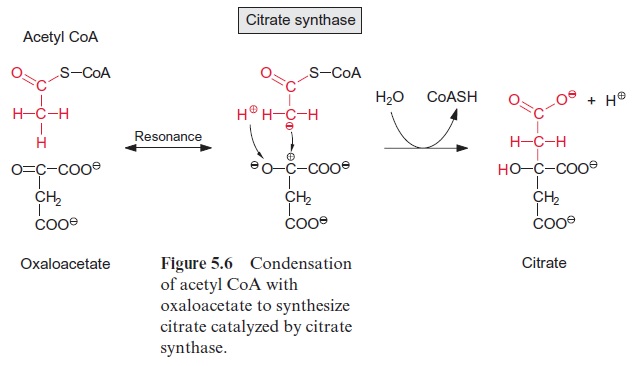
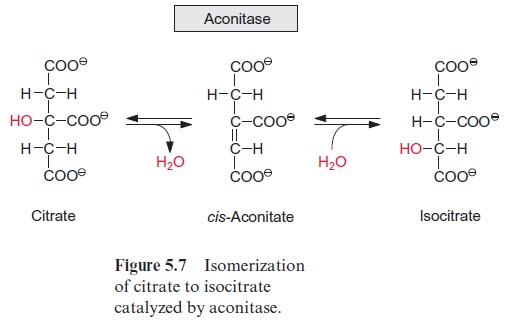
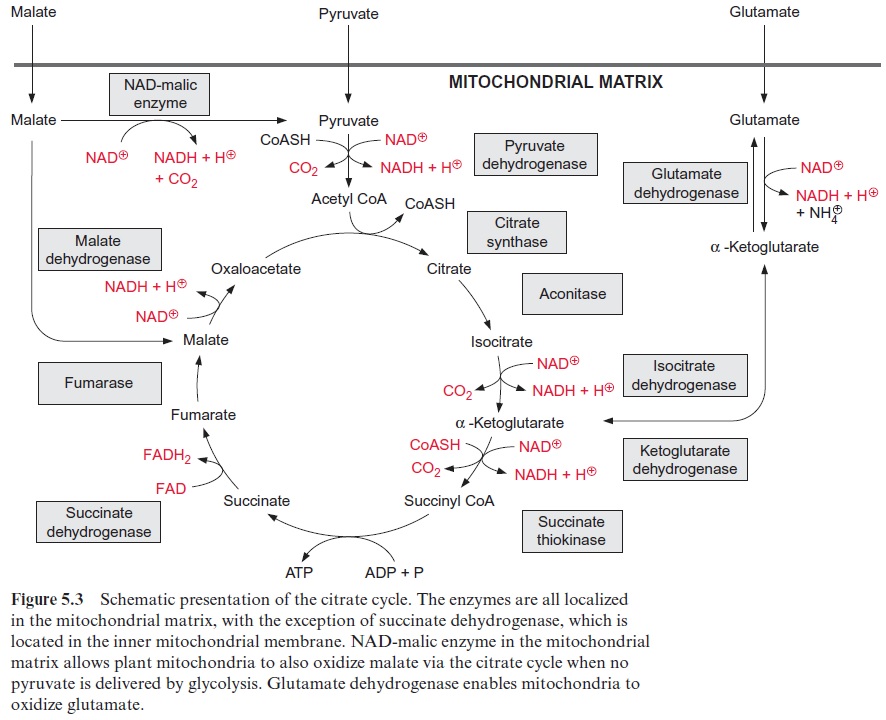
Figure 10.6 shows that glutamate is synthesized in the chloroplasts from α-ketoglutarate, which originates from the mitochondrial citrate cycle (Fig. 10.11). Pyruvate and oxaloacetate are transported from the cytosol to the mitochondria by specific translocators. Pyruvate is oxidized by the pyruvate dehydrogenase complex (see Fig. 5.4), and the acetyl-CoA thus generated condenses with oxaloacetate to citrate (see Fig. 5.6). This citrate can be converted in the mitochondria via the citric acid cycle enzymeaconitase (Fig. 5.7), oxidized further by NAD-isocitrate dehydrogenase (Fig. 5.8), and the resultant -ketoglutarate can be transported into the cytosol by a specific translocator. Often a major part of the citrate produced in the mitochondria is exported to the cytosol and converted there to α-ketoglu-tarate by cytosolic isoenzymes of aconitase and NADP-isocitrate dehydro-genase. Citrate is released from the mitochondria by a specific translocator in exchange for oxaloacetate.
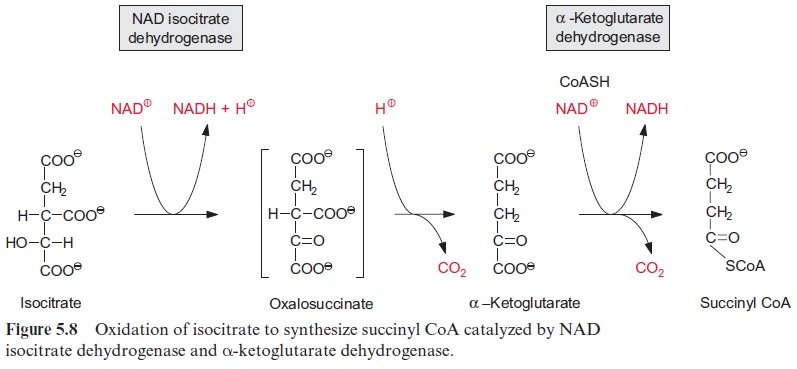
Biosynthesis of proline and arginine
Glutamate is the precursor for the synthesis of proline (Fig. 10.12). Its α-carboxylic group is first converted by a glutamate kinase to an energy-rich phosphoric acid anhydride and is then reduced by NADPH to an aldehyde. The accompanying hydrolysis of the energy-rich phosphate, resembling the reduction of 3-phosphoglycerate to glyceraldehyde 3-phosphate in the Calvin cycle, drives the reaction. A ring is formed by the intramolecular condensation of the carbonyl group with the -amino group. Reduction by NADPH results in the formation of proline.
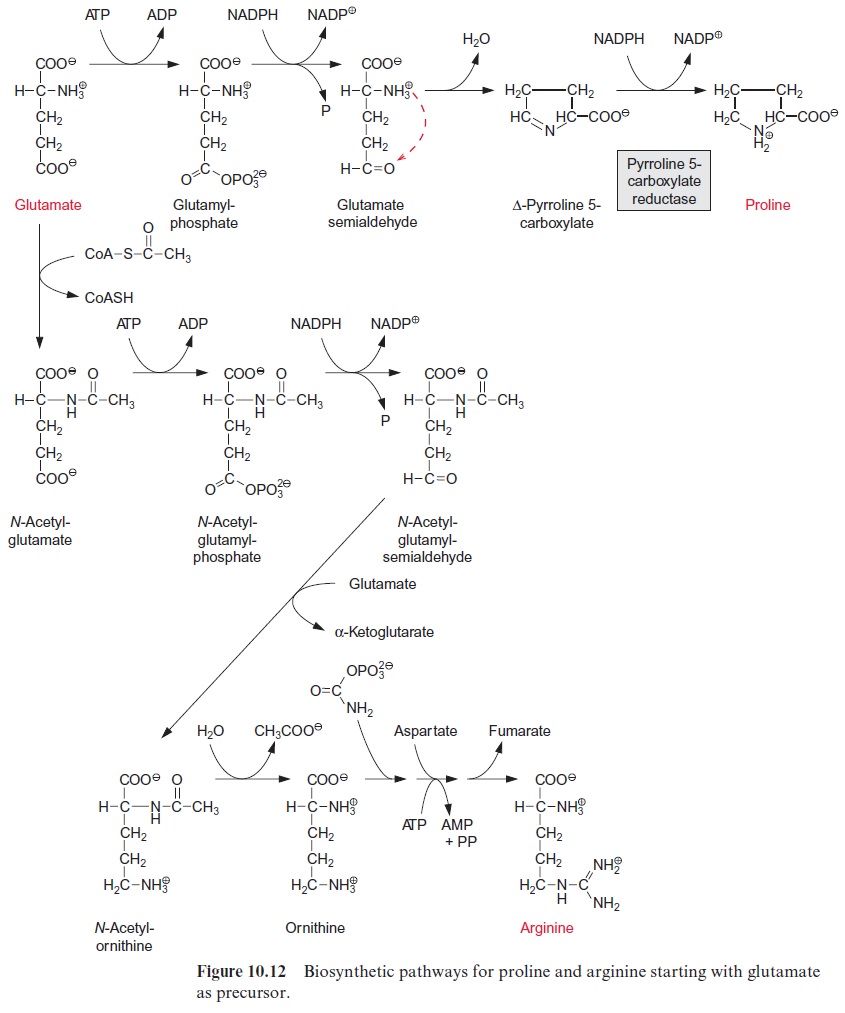
Besides its role as a protein constituent, proline has a special function as a protective substance against dehydration damage in leaves. When exposed to aridity or to a high salt content in the soil (both leading to water stress), many plants accumulate very high amounts of proline in their leaves, in some cases several times the sum of all the other amino acids. It is assumed that the accumulation of proline during water stress is caused by the induction of the synthesis of the enzyme protein of pyrrolin-5-carboxylate reductase.
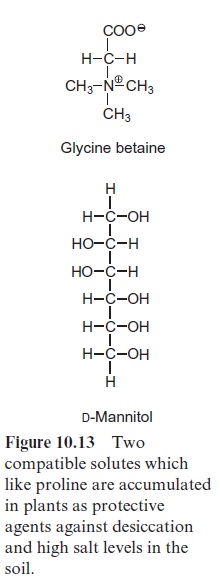
Proline protects a plant against dehydration, because, in contrast to inorganic salts, it has no inhibitory effect on enzymes even at very high con-centrations. Therefore proline is classified as a compatible solute. Other com-patible solutes, formed in certain plants in response to water stress, are sugar alcohols such as mannitol (Fig. 10.13), and betains, consisting of amino acids, such as proline, glycine, and alanine, of which the amino groups are methyl-ated. The latter are termed proline, glycine, and alanine betains. The accu-mulation of such compatible solutes, especially in the cytosol, chloroplasts, and mitochondria, minimizes the damaging effects of water shortage or high salt content of the soil. These compounds also participate as antioxidants in the elimination of reactive oxygen species (ROS) . Water short-age and high salt content of the soil causes an inhibition of CO2 assimilation, resulting in an overreduction of photosynthetic electron transport carriers, which in turn leads to an increased formation of ROS.
In the first step of the synthesis of arginine, the α-amino group of gluta-mate is acetylated by reaction with acetyl-CoA and is thus protected. Subsequently, the β-carboxylic group is phosphorylated and reduced to a semi-aldehyde in basically the same reaction as in proline synthesis. Here the α-amino group is protected and the formation of a ring is not possible. By transamination with glutamate, the aldehyde group is converted to an amino group, and after cleavage of the acetyl residue, ornithine is formed. The conversion of ornithine to arginine (not shown in detail in Fig. 10.12) proceeds in the same way as in the urea cycle of animals by condensation with carbamoyl phosphate to citrulline. An amino group is transferred from aspartate to citrulline, resulting in the formation of arginine and fumarate.
Aspartate is the precursor of five amino acids
Aspartate is formed from oxaloacetate by transamination with glutamate by glutamate-oxaloacetate amino transferase (Fig. 10.14). The synthesis of asparagine from aspartate requires a transitory phosphorylation of the ter-minal carboxylic group by ATP, as in the synthesis of glutamine. In con-trast to glutamine synthesis, however, it is not NH4+ but the amide group of glutamine that usually serves as the amino donor in asparagine synthe-sis. Therefore, the energy expenditure for the amidation of aspartate is twice as high as for the amidation of glutamate. Asparagine is formed to a large extent in the roots, especially when NH4+ is the nitro-gen source in the soil. Synthesis of asparagine in the leaves often plays only a minor role.
For the synthesis of lysine, isoleucine, threonine and methionine, the first two steps are basically the same as for proline synthesis: after phosphoryla-tion by a kinase, theγ-carboxylic group is reduced to a semi-aldehyde. For the synthesis of lysine (not shown in detail in Fig. 10.14), the semi-alde-hyde condenses with pyruvate and, in a sequence of six reactions involving reduction by NADPH and transamination by glutamate, meso-2,6-diami-nopimelate is synthesized and from this lysine arises by decarboxylation.
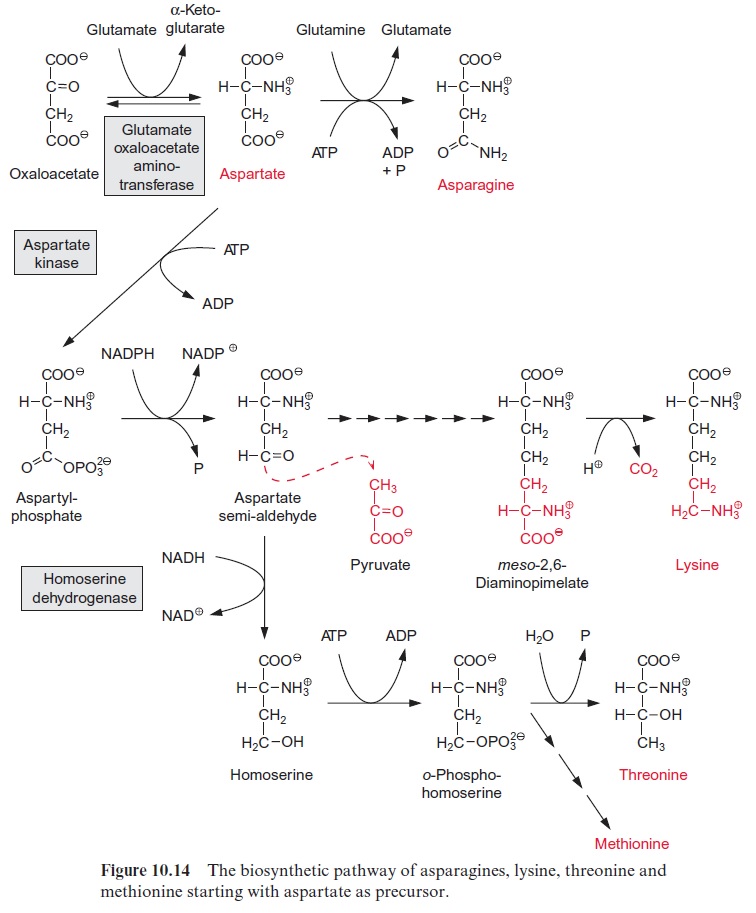
For the synthesis of threonine, the semi-aldehyde is further reduced to homoserine. After phosphorylation of the hydroxyl group by homoser-ine kinase, threonine is formed by isomerization of the hydroxyl group, accompanied by the removal of phosphate. The synthesis of isoleucine from threonine will be discussed in the following paragraph.
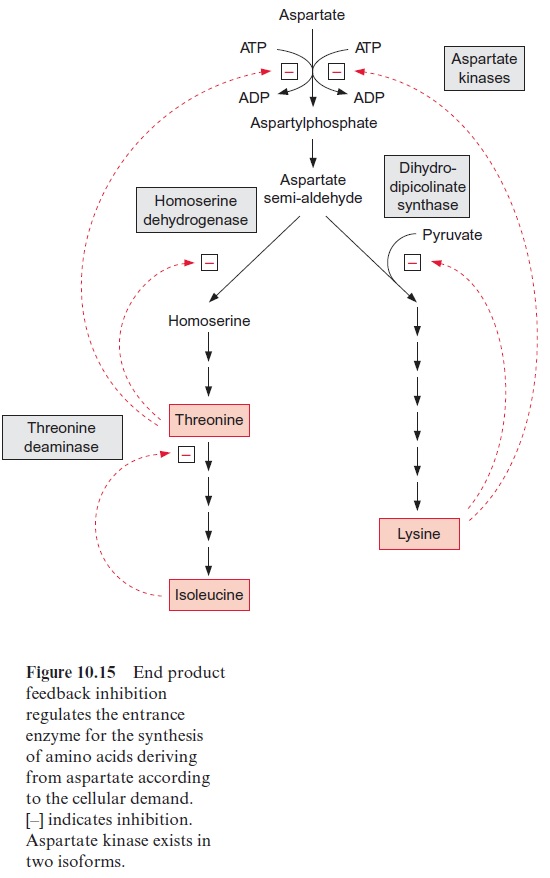
Synthesis of amino acids from aspartate is subject to strong feedback control by its end products (Fig. 10.15). Aspartate kinase, the entrance valve for these pathways, is present in two isoforms. One is inhibited by threonine and the other by lysine. In addition, the reactions that follow aspartate semi-aldehyde at the branch point of both biosynthetic pathways are inhibited by the corresponding end products.
Acetolactate synthase participates in the synthesis of hydrophobic amino acids
Pyruvate can be converted by transamination to alanine (Fig. 10.16A). This reaction plays a special role in C4 metabolism (see Figs. 8.14 and 8.15).
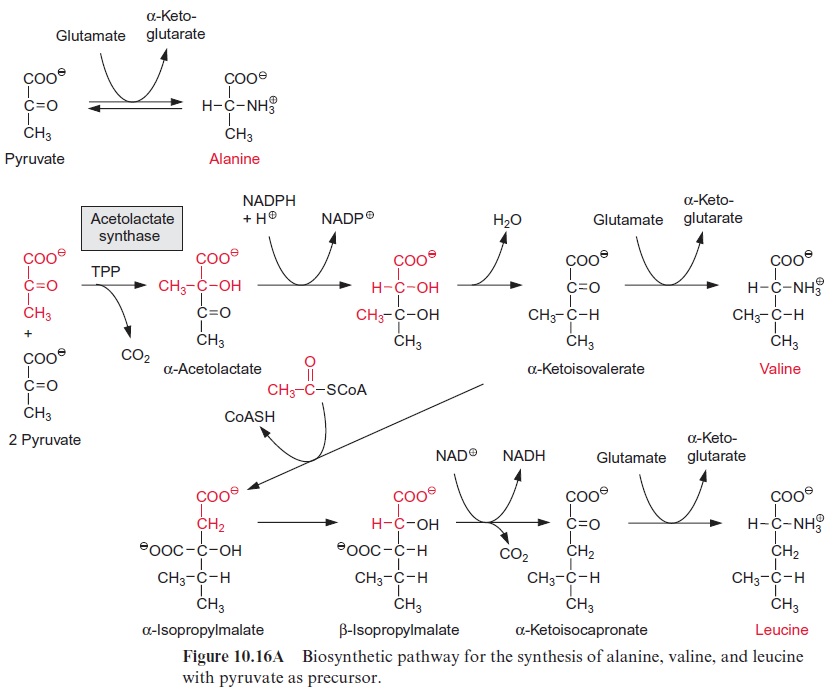
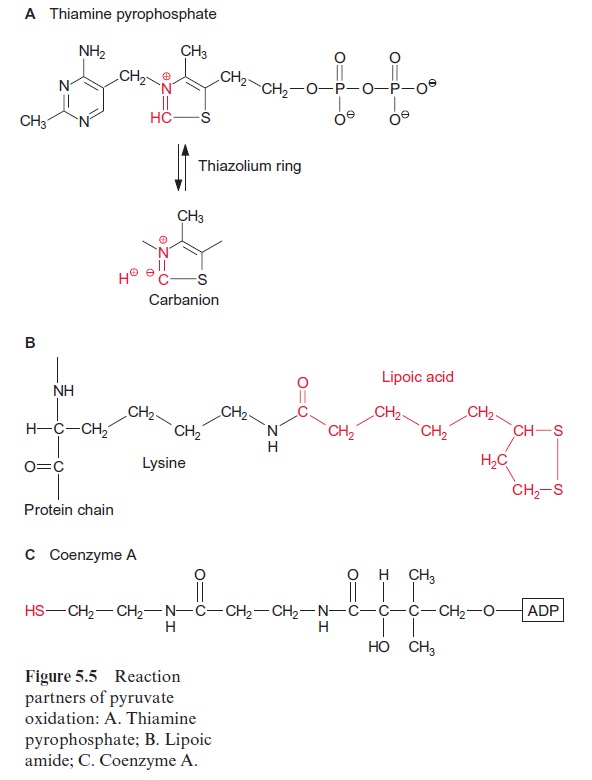
Synthesis of valine and leucine begins with the formation of acetolactate from two molecules of pyruvate. Acetolactate synthase, catalyzing this reac-tion, containsthiamine pyrophosphate (TPP) as prosthetic group. The reac-tion of TPP with pyruvate yields hydroxyethyl-TPP and CO2, in the same way as in the pyruvate dehydrogenase reaction (see Fig. 5.4). The hydrox-yethyl residue is transferred to a second molecule of pyruvate and thus ace tolactate is synthesized. Its reduction and rearrangement and the release of water yields α-ketoisovalerate and a subsequent transamination by gluta-mate produces valine.
The formation of leucine from α-ketoisovalerate proceeds with basically the same reaction sequences as for the synthesis of glutamate from oxaloac-etate shown in Figure 5.3. First, acetyl-CoA condenses with α-ketoisovaler-ate (analogous to the formation of citrate), the product α-isopropylmalate isomerizes (analogous to isocitrate formation), and the β-isopropylmalate thus formed is oxidized by NAD+ with the release of CO2 to α-ketoiso-capronate (analogous to the synthesis of -ketoglutarate by isocitrate dehydrogenase). Finally, in analogy to the synthesis of glutamate, α-ketoi-socapronate is transaminated to leucine.
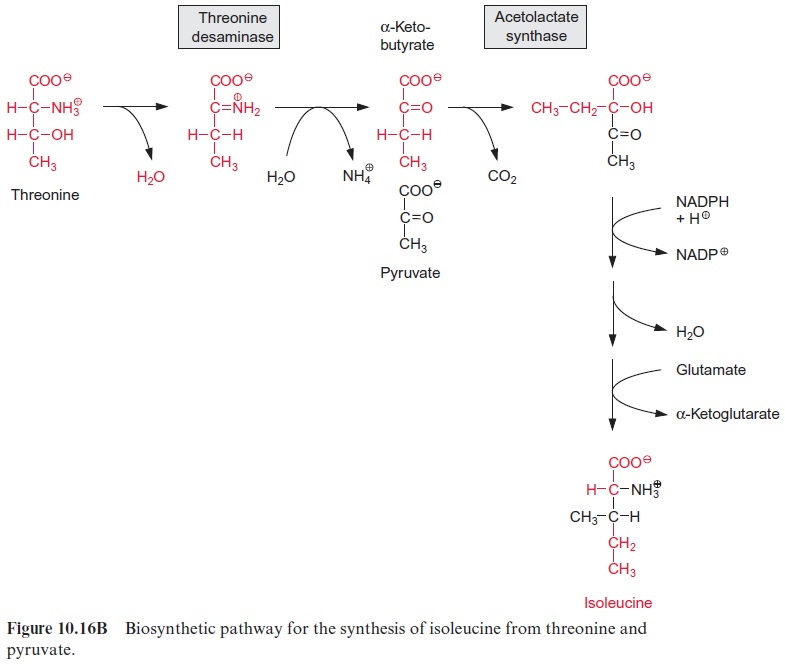
For the synthesis of isoleucine from threonine, the latter is first con-verted by a deaminase to α-ketobutyrate (Fig. 10.16B). Acetolactate syn-thase condenses α-ketobutyrate with pyruvate in a reaction analogous to the synthesis of acetolactate from two molecules of pyruvate (Fig. 10.16A). Further reactions of the synthesis of isoleucine correspond to the reaction sequence of the synthesis of valine.
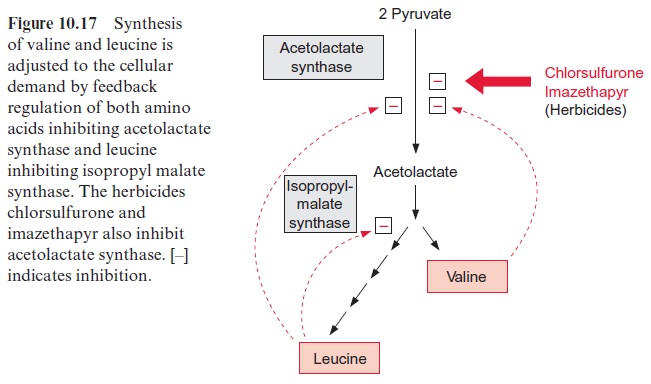
The synthesis of leucine, valine, and isoleucine is also subject to feed-back control by the end products. Isopropylmalate synthase is inhibited by leucine (Fig. 10.17) and threonine deaminase is inhibited by isoleucine (Fig. 10.15). The first enzyme, acetolactate synthase (ALS), is inhibited by valine and leucine. Sulfonyl ureas (e.g., chlorsulfurone) and imidazolinones (e.g., imazethapyr) (Fig. 10.18), are very strong inhibitors of ALS, since these compounds bind to the pyruvate binding site. A concentration as low as 10–9 mol/L of chlorsulfurone is sufficient to inhibit ALS by 50%. Since the pathway for the formation of valine, leucine, and isoleucine is present only in plants and microorganisms, the aforementioned inhibitors are suitable to kill specifically plants and are therefore used as efficient herbicides .
Chlorsulfurone (trade name Glean, DuPont) is applied as a selective her-bicide in the cultivation of cereals, and Imazethapyr (Pursuit, American Cyanamide Co.) is used for protecting soybeans. The application of these herbicides resulted in naturally evolved mutants of maize, soybean, rape-seed, and wheat, which are resistant to sulfonyl ureas or imidazolinones, or even to both herbicides. In each case, a mutation was found in the gene for acetolactate synthase, making the enzyme insensitive to the herbicides with-out affecting its enzyme activity. By crossing these mutants with other lines, herbicide-resistant varieties have been bred and are, in part, already commer-cially cultivated.
Aromatic amino acids are synthesized via the shikimate pathway
Precursors for the formation of aromatic amino acids are erythrose 4-phosphate and phosphoenolpyruvate. These two compounds condense to form cyclic dehydrochinate accompanied by the liberation of both phos-phate groups (Fig. 10.19). Following the removal of water and the reduc-tion of the carbonyl group, shikimate is formed. After protection of the 3’ -hydroxyl group by phosphorylation, the 5’ -hydroxyl group of shikimate reacts with phosphoenolpyruvate to synthesize the enolether 5’ -enolpyru-vyl shikimate-3-phosphate (EPSP). From this chorismate is formed by the removal of phosphate and represents a branch point for two biosynthetic pathways:
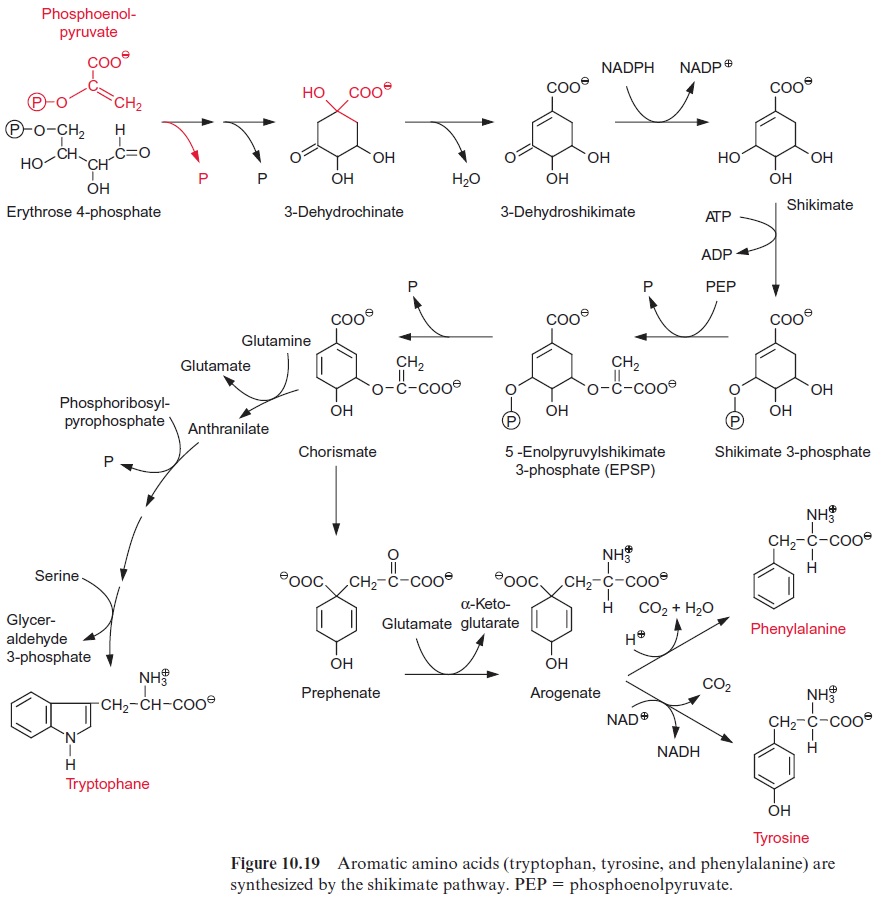
1. Tryptophan is synthesized via four reactions, which are not discussed in detail here.
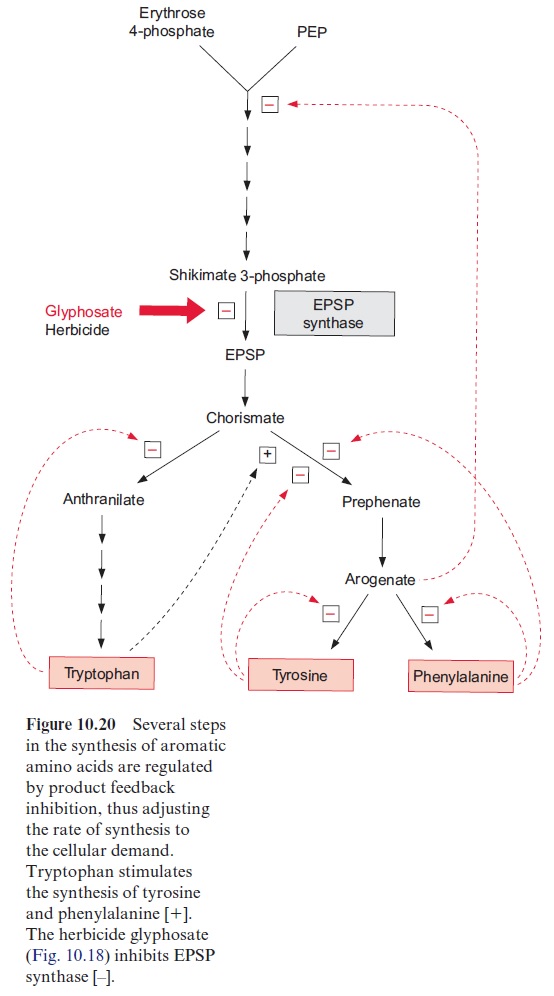
2. Prephenate is produced by a rearrangement, in which the side chain is transferred to the 1’ -position of the ring, and arogenate is formed after transamination of the keto group. Removal of water results in the for-mation of the third double bond and phenylalanine is synthesized by decarboxylation. Oxidation of arogenate by NAD+ , accompanied by a decarboxylation, results in the formation of tyrosine. According to recent results, the enzymes of the shikimate pathway are located exclu-sively in theplastids. The synthesis of aromatic amino acids is also con-trolled at several steps in the pathway by the end products (Fig. 10.20).
Glyphosate acts as a herbicide
Glyphosate (Fig. 10.18), a structural analogue of phosphoenolpyruvate, is a very strong inhibitor of EPSP synthase. Glyphosate inhibits specifically the synthesis of aromatic amino acids but has only a low effect on other phosphoenolpyruvate metabolizing enzymes (e.g., pyruvate kinase or PEP carboxykinase). Interruption of the shikimate pathway by glyphosate has a lethal effect on plants. Since the shikimate pathway is not present in ani-mals, glyphosate (under the trade name Roundup, Monsanto) is used as a selective herbicide . Due to its simple structure glyphosate is relatively quickly degraded by soil bacteria. Glyphosate is the herbicide with the highest sales worldwide. Genetic engineering successfully created glyophosate-resistant crop plants , which allows an efficient weed control in the presence of such transgenic crop plants.
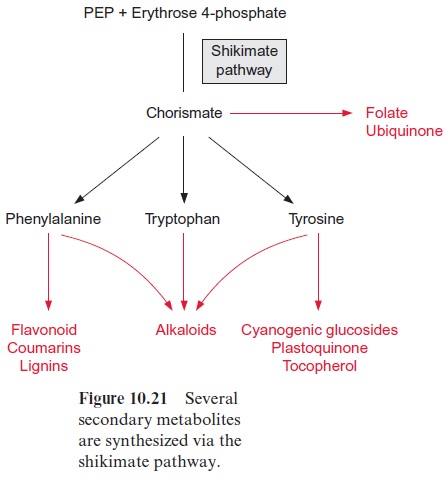
A large proportion of the total plant matter can be formed by the shikimate pathway
The shikimate pathway is not restricted to the generation of amino acids for protein biosynthesis. It also provides precursors for a large variety of other compounds (Fig. 10.21) formed by plants in large quantities, particularly phenylpropanoids such as flavonoids and lignin . These prod-ucts can amount to a high proportion of the total cellular matter, in some plants up to 50% of the dry matter. Therefore the shikimate pathway can be regarded as one of the pronounced biosynthetic pathways of plants.
Related Topics