Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Dispensing Biotechnology Products: Handling, Professional Education, and Product Information
The Pharmacist and Handling of Biotech Drugs
The Pharmacist and Handling of Biotech Drugs
The pharmacist is responsible for the storage, preparation and
dispensing of biotechnology drugs as well as patient education regarding the
use of these products. In many cases, pharmacists must have additional training
in order to be prepared for this role. This is especially true for pharmacists
who practice in the ambulatory care setting since these products are
increasingly available for administration by the patient in the home.
Pharmacies of the future may stock pumps, patches, timed-release tablets,
liposomes, implants, and vials of tailored monoclonal antibodies. With gene
therapy and gene splicing on the horizon, it is possible that the pharmacist
may eventually prepare and dispense gene therapy pro-ducts tailored for
specific patients.
This chapter will discuss the general principles that pharmacists need
to understand about storage, handling, preparation, administration of biotech
pro-ducts and issues related to outpatient/home care. Specific examples will be
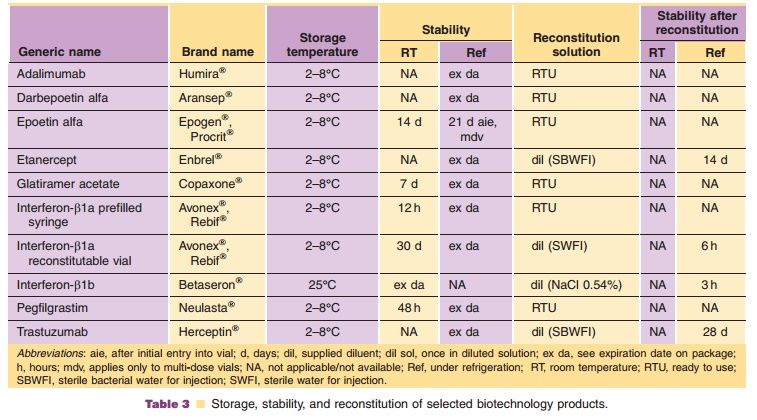
discussed for illustrative purposes. Table 3 lists selected products along with
specific handling requirements for each. For specific products or recent
updates, contact the manufacturer. For additional information regarding drug
handling and preparation, pharmacist may consult the following publications:
American Hospital Formulary Drug Information and the King Guide to Parenteral
Admixture (Catania, 2006). In addition to hardcover publications with frequent
updates, both of these references are available on-line at www.ashp.org/ ahfs/
and www.kingguide.com. Pharmacy benefits management companies usually own
specialty phar-macy companies and provide valuable information via their
websites. Two well-known companies are RxSolutions and Caremark. 28 days
although refrigeration is recommended. Since most of these products need to be
kept at refrigerated temperatures (as discussed below), some pharmacies may
need to increase cold storage space in order to accommodate the storage needs.
Related Topics