Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Dispensing Biotechnology Products: Handling, Professional Education, and Product Information
Outpatient/Home Care Issues of Biotechnology Products
OUTPATIENT/HOME CARE ISSUES
As mentioned previously, the management of patients in the outpatient and home settings is now an accepted aspect of health care delivery. The use of biotech products outside the hospital is no exception to this trend. Home infusion and specialty pharmacy services dispense all forms of parenteral and enteral products including biotech drugs. These pharmacies have grown exponentially in the last twenty years due to cost savings for third party payers, technological advances that allow these services to occur in the home, and patient preference to be treated at home rather than an in-patient setting.
Patient Assessment and Education
Before a patient can be a candidate for home therapy, an assessment of the patient’s capabilities must occur. The patient, family member, or caregiver will need to be able to administer the medication and comply with all of the storage, handling, and preparation require-ments. If the patient is incapable, then a caregiver (usually a relative, spouse, or friend) needs to be recruited to assist the patient. The pharmacy staff or other health professional may also make home visits to assist the patient in these tasks. The use of aseptic technique is usually new to the patients and in some cases may be overwhelming. The healthcare provider must be sure that the patient or caregiver is competent and willing to follow these procedures. Self-instruc-tional guides on specific products may be available from the manufacturer, and if so, should be provided to the patient providing they have the proper equipment for viewing.
Proper storage facilities will need to be available in the patient’s home as well as a clean area for preparation and administration. Ideally, the patient will be able to prepare each dose immediately prior to the time of administration. If this is not possible, the pharmacy will have to prepare pre-filled syringes and provide appropriate storage and handling require-ments to the patient. The patient will also need to be educated regarding the proper handling of the syringes as well as other required supplies and materials such as needles, syringes, alcohol wipes, etc. Proper disposal of these hazardous wastes must also be reviewed. Specific issues related to patient teaching include rotating injection sites, product handling, drug storage including transporting and traveling with biotech drugs, expiration dates, refrig-eration, cleansing the injection site with alcohol, disposal of needles and syringes, potential adverse effects, and expected therapeutic outcomes.
Monitoring
For patients who receive biotech drug therapy in the home, it is particularly important that close patient monitoring occurs. This will require frequent phone calls to the patient and periodic home visits. Monitoring parameters should include adverse events, progress to expected outcomes, assessment of administration technique, review of storage and handling procedures, and adherence to aseptic technique.
Reimbursement
Reimbursement issues include third party billing information and availability of forms, cost sharing programs that limit the annual cost of therapy, financial assistance programs for patients who would otherwise have difficulty paying for therapy, and reimbursement assurance programs that are designed to remove reimbursement barriers when reimburse-ment has been denied. and is subject to practice location. This discussion will deal only with the availability of information to pharmacists to appropriately handle reimbursement for products and services in the United States.
Pharmacists need to know current third party payment policies including those conditions under which insurance companies will disallow claims. Some examples include off-label prescribing or administration of the product in the home rather than administration in a hospital or physician’s office. Prior authorization is usually required particularly with managed care or prepaid plans. Manufacturers will often assist the patient by contacting the carrier to verify coverage, providing sample prior-approval letters, and following up on claims to determine the claim’s status and continuing to follow the case until it is resolved.
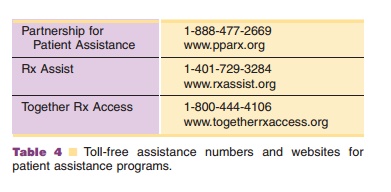
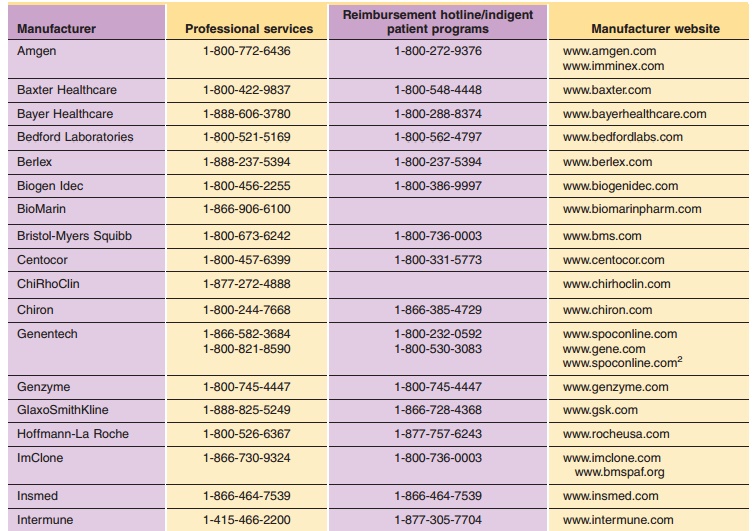
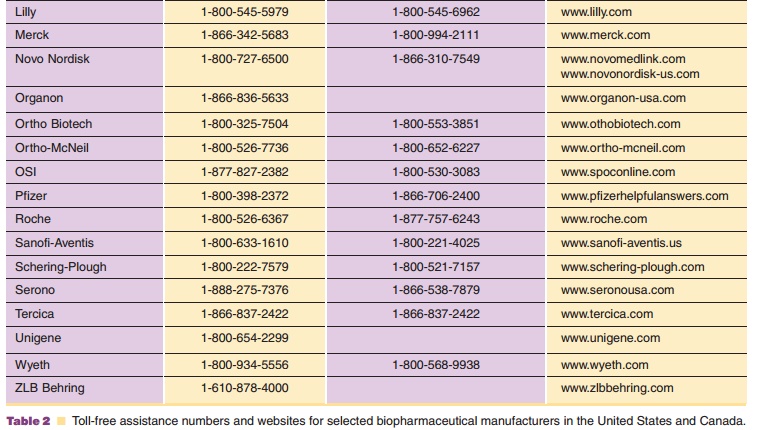
Manufacturers can also provide information that may convince the third-party payer to reconsider a denied claim. Some companies will intervene with the third party payer to evaluate the case for denial, provide additional clinical documentation or coding information, and will follow the appeal to conclusion. Pharmacists can act as facilitators to get qualified patients enrolled in programs to provide free medica-tion to those who have insufficient insurance coverage or are otherwise unable to purchase the therapy. Manufacturers’ websites and toll-free numbers for reimbursement issues are provided in Table 2. Websites and toll-free numbers for some of the patient assistance programs are provided in Table 4. The Partnership for Patient Assistance website provides information on a variety of patient assistance programs as well as the requirements to qualify for various programs.
Related Topics