Chapter: Biochemistry: The Citric Acid Cycle
The Individual Reactions of the Citric Acid Cycle
The Individual Reactions of the
Citric Acid Cycle
The reactions of the citric acid cycle proper and the enzymes that
catalyze them are listed in Table 19.1. We shall now discuss each of these
reactions in turn.
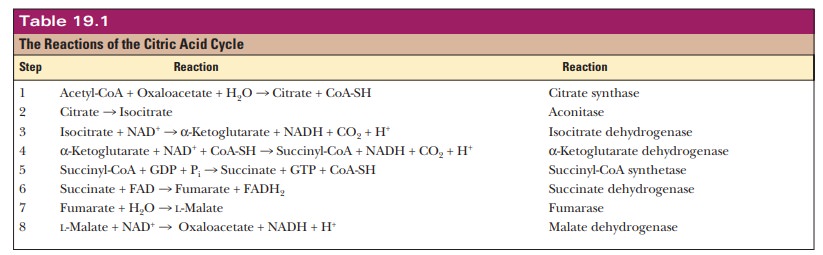
Step 1.Formation of
CitrateThe first step of the citric acid cycle is thereaction
of acetyl-CoA and oxaloacetate to form citrate and CoA-SH. This reaction is
called a condensation because a new carbonŌĆōcarbon bond is formed. The
condensation reaction of acetyl-CoA and oxaloacetate to form citryl-CoA takes
place in the first stage of the reaction. The condensation is followed by the
hydrolysis of citryl-CoA to give citrate and CoA-SH.
The
reaction is catalyzed by the enzyme citrate
synthase, originally called ŌĆ£condensing enzyme.ŌĆØ A synthase is an enzyme
that makes a new covalent bond during the reaction, but it does not require the
direct input of ATP. It is an exergonic reaction ( ŌłåG┬░' = ŌĆō32.8 kJ molŌĆō1 = ŌĆō7.8 kcal molŌĆō1)
because the hydrolysis of a thioester releases energy. Thioesters are
considered high-energy compounds.
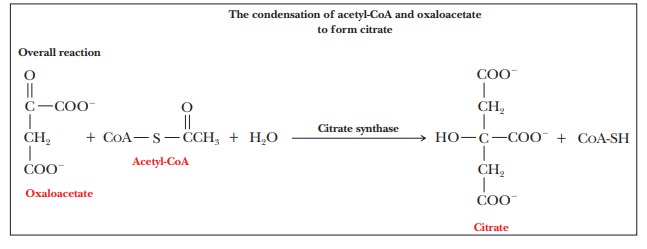
Step 2.Isomerization of
Citrate to IsocitrateThe second reaction of the citricacid cycle, the one catalyzed
by aconitase, is the isomerization of citrate to isocitrate. The enzyme
requires Fe2+. One of the most interesting features of the reaction
is that citrate, a symmetrical (achiral) compound, is converted to isocitrate,
a chiral compound, a molecule that cannot be superimposed on its mirror image.
It is
often possible for a chiral compound to have several different isomers.
Isocitrate has four possible isomers, but only one of the four is produced by
this reaction. Aconitase, the enzyme that catalyzes the conversion of citrate
to isoci-trate, can select one end of the citrate molecule in preference to the
other.
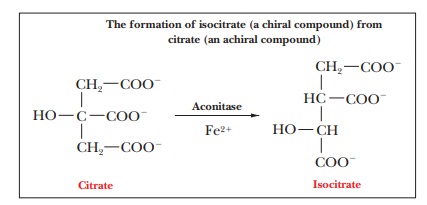
This
type of behavior means that the enzyme can bind a symmetrical substrate in an
unsymmetrical binding site. We mentioned that this possibility exists, and here
we have an example of it. The enzyme forms an unsymmetrical three-point
attachment to the citrate molecule (Figure 19.6). The reaction proceeds by
removal of a water molecule from the citrate to produce cis-aconitate, and then water is added back to the cis-aconitate to give isocitrate.
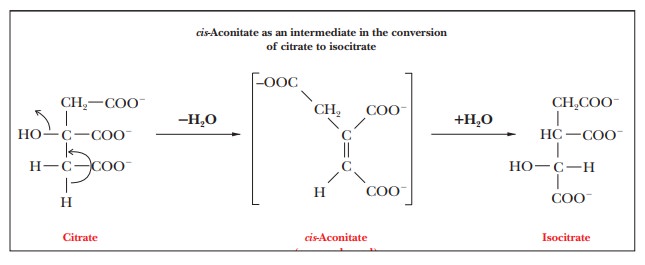
The
intermediate, cis-aconitate, remains
bound to the enzyme during the course of the reaction. There is some evidence that
the citrate is complexed to the Fe(II) in the active site of the enzyme in such
a way that the citrate curls back on itself in a nearly circular conformation.
Several authors have been unable to resist the temptation to call this
situation the ŌĆ£ferrous wheel.ŌĆØ
Step 3.Formation of
-Ketoglutarate and CO2ŌĆöFirst OxidationThe thirdstep in the citric acid cycle is the oxidative
decarboxylation of isocitrate to ╬▒-ketoglutarate
and carbon dioxide. This reaction is the first of two oxidativedecarboxylations
of the citric acid cycle; the enzyme that catalyzes it is isocitrate dehydrogenase. The reaction takes place in two steps
(Figure 19.7).First, isocitrate is oxidized to oxalosuccinate, which remains
bound to the enzyme. Then oxalosuccinate is decarboxylated, and the carbon
dioxide and ╬▒-ketoglutarate are released.
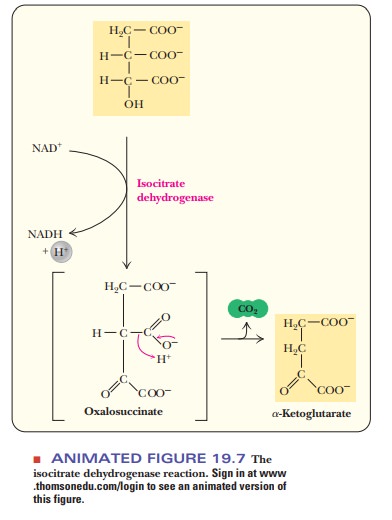
This is the first of the reactions in which NADH is produced. One
molecule of NADH is produced from NAD+ at this
stage by the loss of two electrons in the oxidation. As we saw in our
discussion of the pyruvate dehydrogenase com-plex, each NADH produced leads to
the production of 2.5 ATP in later stages of aerobic metabolism. Recall also
that there will be two NADH, equivalent to five ATP for each original molecule
of glucose.
Step 4.Formation of
Succinyl-CoA and CO2ŌĆöSecond OxidationThesecond oxidative decarboxylation takes place in Step 4 of the
citric acid cycle, in which carbon dioxide and succinyl-CoA are formed from ╬▒-ketoglutarate and CoA.
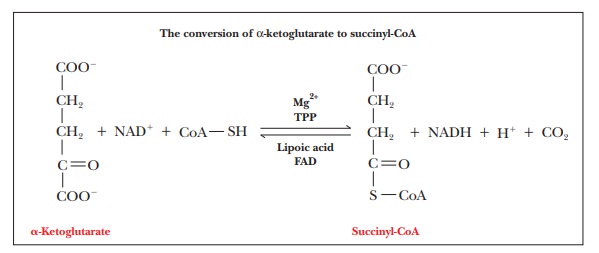
This
reaction is similar to the one in which acetyl-CoA is formed from pyruvate,
with NADH produced from NAD+. Once again, each NADH eventually gives
rise to 2.5 ATP, with five ATP from each original molecule of glucose.
The
reaction occurs in several stages and is catalyzed by an enzyme system called
the ╬▒-ketoglutarate dehydrogenase
complex, which
is very similar to the pyruvate dehydrogenase complex. Each of these
multienzyme systems consists of three enzymes that catalyze the overall
reaction. The reaction takes place in several steps, and there is again a
requirement for thiamine pyrophosphate (TPP), FAD, lipoic acid, and Mg2+.
This reaction is highly exergonic ( ŌłåG┬░'
= ŌĆō33.4 kJ molŌĆō1 = ŌĆō8.0 kcal molŌĆō1), as is the one
catalyzed by pyruvate dehydrogenase.
At this
point, two molecules of CO2 have been produced by the oxidative
decarboxylations of the citric acid cycle. Removal of the CO2 makes
the citric acid cycle irreversible in vivo, although in vitro each separate
reaction is revers-ible. One might suspect that the two molecules of CO2
arise from the two car-bon atoms of acetyl-CoA. Labeling studies have shown
that this is not the case, but a full discussion of this point is beyond the
scope of this text.
The two
CO2 arise from carbon atoms that were part of the oxaloacetate with
which the acetyl group condensed. The carbons of this acetyl group are
incorporated into the oxaloacetate that will be regenerated for the next round
of the cycle. The release of the CO2 molecules has a profound
influence on mammalian physiology, as will be discussed later. We should also
mention that the ╬▒-ketoglutarate dehydrogenase complex reaction
is the third one in which we have encountered an enzyme that requires TPP.
Step 5.Formation of
SuccinateIn the next step of the cycle, the thioester bondof succinyl-CoA is hydrolyzed
to produce succinate and CoA-SH; an accompanying reaction is the
phosphorylation of GDP to GTP. The whole reaction is catalyzed by the enzyme succinyl-CoA synthetase. A synthetase
is an enzyme that creates a new covalent bond and requires the direct input of
energy from a high-energy phosphate. Recall that we met a synthase (citrate
synthase) earlier. The difference between a synthase and a synthetase is that a
synthase does not require energy from phosphate-bond hydrolysis, whereas a
synthetase does. In the reaction mechanism, a phosphate group covalently bonded
to the enzyme is directly transferred to the GDP. The phosphorylation of GDP to
GTP is endergonic, as is the corresponding ADP-to-ATP reaction ( ŌłåG┬░' = 30.5 kJ molŌĆō1 = 7.3
kcal molŌĆō1).
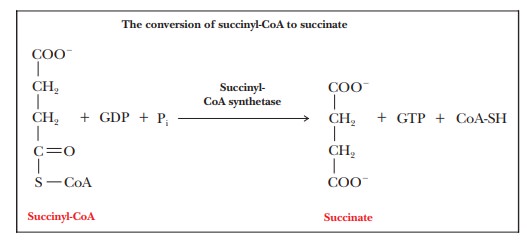
The
energy required for the phosphorylation of GDP to GTP is provided by the
hydrolysis of succinyl-CoA to produce succinate and CoA. The free energy of
hydrolysis ( ŌłåG┬░') of succinyl-CoA is
ŌĆō33.4 kJ molŌĆō1 (ŌĆō8.0 kcal molŌĆō1). The overall reaction is
slightly exergonic ( ŌłåG┬░' = ŌĆō3.3 kJ
molŌĆō1 = ŌĆō0.8 kcal molŌĆō1) and, as a result, does not
contribute greatly to the overall production of energy by the mitochondrion.
Note that the name of the enzyme describes the reverse reaction. Succinyl-CoA
synthetase would produce succinyl-CoA while spending an ATP or another
high-energy phosphate. This reaction is the opposite of that.
The
enzyme nucleosidediphosphate kinase
catalyzes the transfer of a phos-phate group from GTP to ADP to give GDP and
ATP.
GTP +
ADP - > GDP + ATP
This
reaction step is called substrate-level phosphorylation to distinguish it from
the type of reaction for production of ATP that is coupled to the electron
transport chain. The production of ATP in this reaction is the only place in
the citric acid cycle in which chemical energy in the form of ATP is made
available to the cell. Except for this reaction, the generation of ATP
characteristic of aerobic metabolism is associated with the electron transport
chain. About 30 to 32 molecules of ATP can be obtained from the oxidation of a
single molecule of glucose by the combination of anaerobic and aerobic
oxidation, compared with only two molecules of ATP produced by anaerobic
glycolysis alone. The combined reactions that occur in mitochondria are of
great importance to aerobic organisms.
In the
next three steps in the citric acid cycle (Steps 6 through 8), the four-carbon
succinate ion is converted to oxaloacetate ion to complete the cycle.
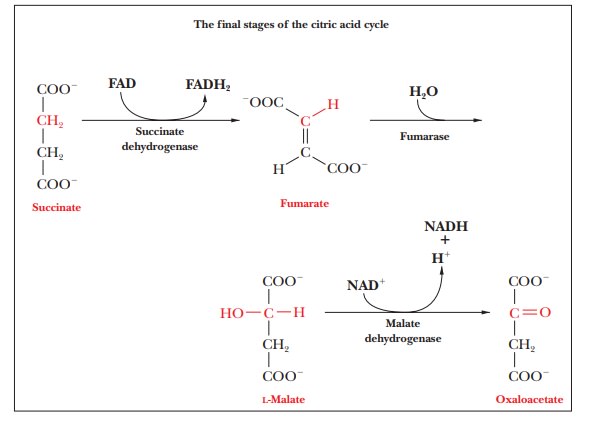
Step 6.Formation of
FumarateŌĆöFAD-Linked OxidationSuccinate isoxidized to fumarate, a
reaction that is catalyzed by the enzyme succinatedehydrogenase.
This enzyme is an integral protein of the inner mitochondrialmembrane. The
other individual enzymes of the citric acid cycle are in the mitochondrial
matrix. The electron acceptor, which is FAD rather than NAD+, is covalently bonded to the enzyme; succinate
dehydrogenase is also called a flavoprotein because of the presence of FAD with
its flavin moiety. In the succinate dehydrogenase reaction, FAD is reduced to
FADH2 and succinate is oxidized to
fumarate.
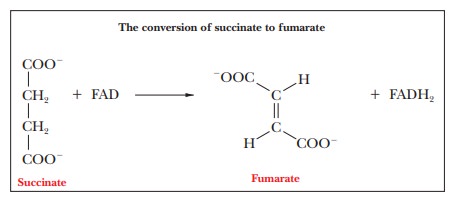
The overall reaction is
Succinate + E-FAD - > Fumarate + E-FADH2
The E-FAD and E-FADH2 in the equation indicate
that the electron accep-tor is covalently bonded to the enzyme. The FADH2 group
passes electrons on to the electron transport chain, and eventually to oxygen,
and gives rise to 1.5 ATP, rather than 2.5, as is the case with NADH.
Succinate dehydrogenase contains iron atoms but does not contain a
heme group; it is referred to as a nonheme
iron protein or an iron-sulfur
protein. The latter name refers to the fact that the protein contains
several clusters that con-sist of four atoms each of iron and of sulfur.
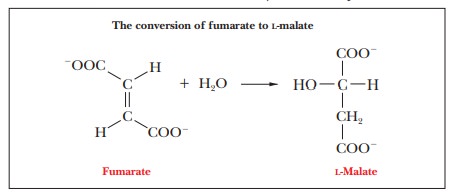
Step 7.Formation ofL-MalateIn Step 7, which is catalyzed by the enzymefumarase, water is added across the double bond of fumarate in a
hydrationreaction to give malate. Again, there is stereospecificity in the
reaction. Malate has two enantiomers, L- and D-malate, but only
L-malate is
produced.
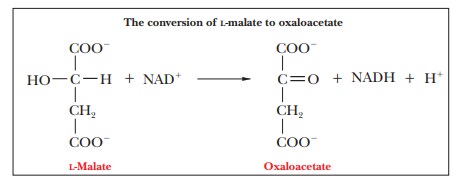
Step 8.Regeneration of
OxaloacetateŌĆöFinal Oxidation StepMalateis oxidized
to oxaloacetate, and another molecule of NAD+is reduced to NADH. This reaction is catalyzed by the enzyme malate dehydrogenase. The oxaloacetate
can then react with another molecule of acetyl-CoA to start another round of
the cycle.
The oxidation of pyruvate by the pyruvate dehydrogenase complex and
the citric acid cycle results in the production of three molecules of CO2. As a
result of these oxidation reactions, one molecule of GDP is phosphorylated to
GTP, one molecule of FAD is reduced to FADH2, and
four molecules of NAD+ are reduced to NADH. Of the
four molecules of NADH produced, three come from the citric acid cycle, and one
comes from the reaction of the pyruvate dehydrogenase complex. The overall
stoichiometry of the oxidation reactions is the sum of the pyruvate
dehydrogenase reaction and the citric acid cycle. Note that only one
high-energy phosphate, GTP, is produced directly
from the citric acid cycle, but many more ATP will arise from reoxidation of
NADH and FADH2.
Pyruvate dehydrogenase
complex:
Pyruvate + CoA-SH + NAD+ - > Acetyl-CoA
+ NADH + CO2 + H+
Citric acid cycle:
Acetyl-CoA + 3NAD+ + FAD + GDP + Pi + 2H2O ->
2CO2 + CoA-SH + 3NADH + 3H+ + FADH2 + GTP
Overall reaction:
Pyruvate + 4NAD+ + FAD + GDP + Pi + 2H2O ->
3CO2 + 4NADH + FADH2 + GTP + 4H+
Eventual ATP production per
pyruvate:
4NADH - > 10ATP (2.5ATP for each NADH)
1FADH - > 1.5ATP (1.5ATP for each FADH2)
1GTP - > 1ATP
Total 12.5 ATP per pyruvate or 25 ATP per glucose
There were also two ATP produced per glucose in glycolysis and two
NADH, which will give rise to another five ATP (seven more ATP total).
At this point, we would do well to recapitulate what we have said
about the citric acid cycle (see Figure 19.3). When studying a pathway such as
this, we might learn many details but also be able to see the big picture. The
entire pathway is shown with the enzyme names outside the circle. The most
impor-tant reactions can be identified by those that have important cofactors
(NADH, FADH2, GTP). Also important are the steps where CO2 is
given off.
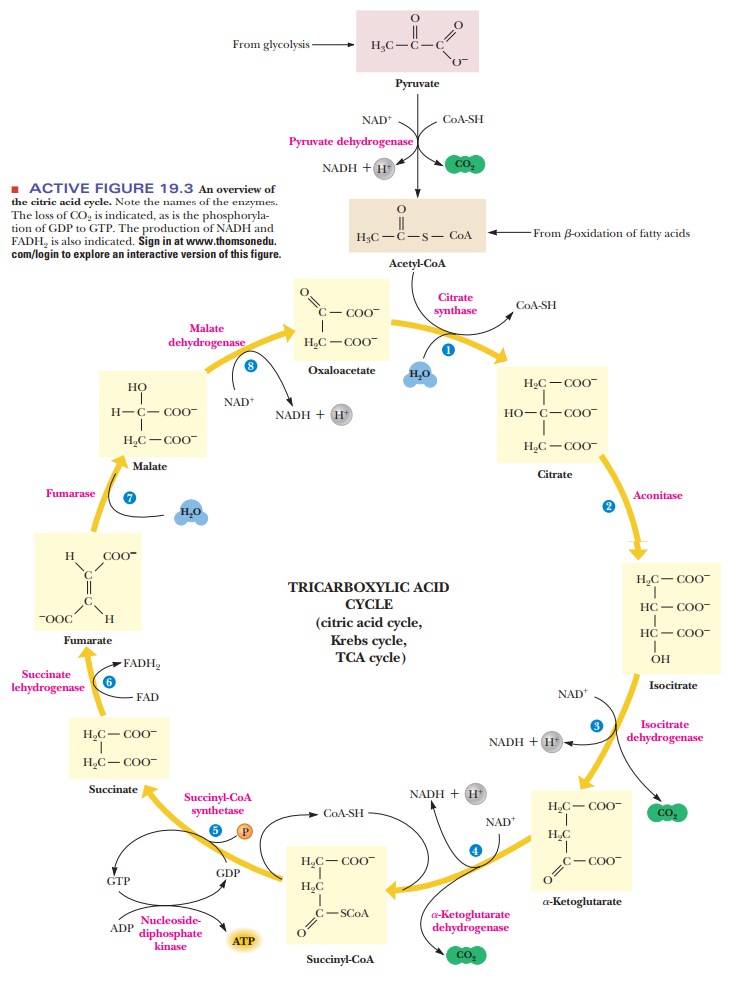
These important reactions also play a large role in the cycleŌĆÖs
contribution to our metabolism. One purpose of the cycle is to produce energy.
It does that by producing GTP directly and by producing reduced electron
carriers (NADH and FADH2). The three decarboxylations
mean that for every three carbons entering as pyruvate, three carbons are
effectively lost during the cycle, a fact that has many implications to our
metabolism.
Summary
In the citric acid cycle and the pyruvate dehydrogenase reaction,
one molecule of pyruvate is oxidized to three molecules of carbon dioxide as a
result of oxidative decarboxylations
The oxidations are accompanied by reductions. Four NAD+ are
reduced to NADH, and one FAD to FADH2; in
addition, one GDP is phosphory-lated to GTP.
Related Topics