Chapter: Biochemistry: The Citric Acid Cycle
Energetics and Control of the Citric Acid Cycle
Energetics and Control of the
Citric Acid Cycle
The
reaction of pyruvate to acetyl-CoA is exergonic, as we have seen ( ŌłåG┬░' = ŌĆō33.4 kJ molŌĆō1 = ŌĆō8.0
kcal molŌĆō1). The citric acid cycle itself is also exergonic ( ŌłåG┬░' = ŌĆō44.3 kJ molŌĆō1 = ŌĆō10.6
kcal molŌĆō1), and you will be asked in Question 38 to confirm this
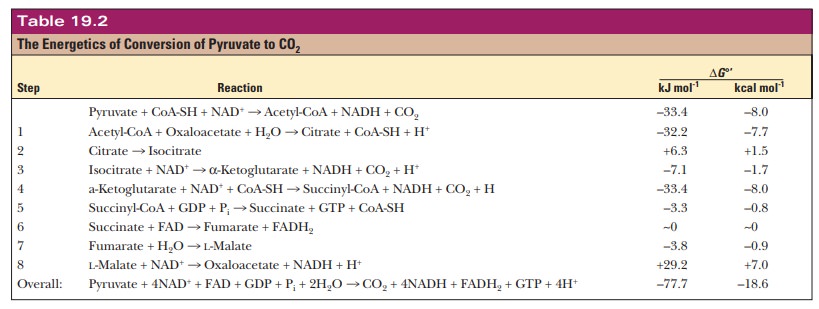
point. The standard free-energy changes for the individual reactions are listed
in Table 19.2. Of the individual reactions of the cycle, only one is strongly
endergonic: the oxidation of malate to oxaloacetate ( ŌłåG┬░' = +29.2 kJ molŌĆō1 = +7.0 kcal molŌĆō1).
This endergonic reaction is, however, coupled to one of the strongly exergonic
reactions of the cycle, the condensation of acetyl-CoA and oxaloacetate to
produce citrate and coenzyme A ( ŌłåG┬░'
= ŌĆō32.2 kJ molŌĆō1 = ŌĆō7.7 kcal molŌĆō1). (Recall that these
values for the free-energy changes refer to standard conditions. The effect of
concentrations of metabolites in vivo can change matters drastically.) In
addition to the energy released by the oxidation reactions, there is more
release of energy to come in the electron transport chain. When the four NADH
and single FADH2 produced by the pyruvate dehydrogenase complex and
citric acid cycle are reoxidized by the electron transport chain, considerable
quantities of ATP are produced. Controlof
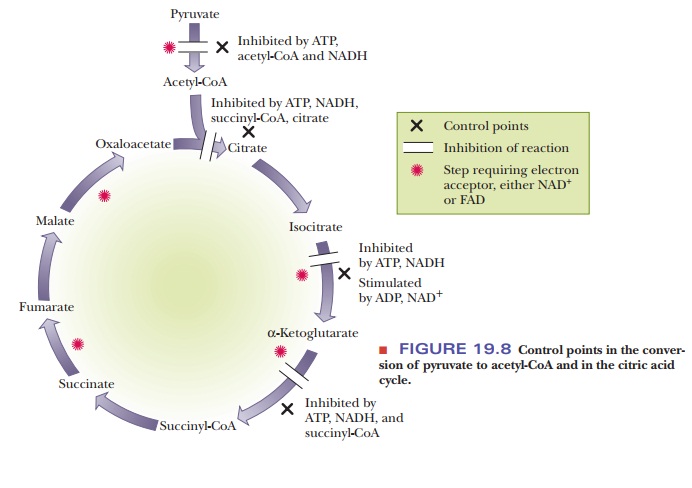
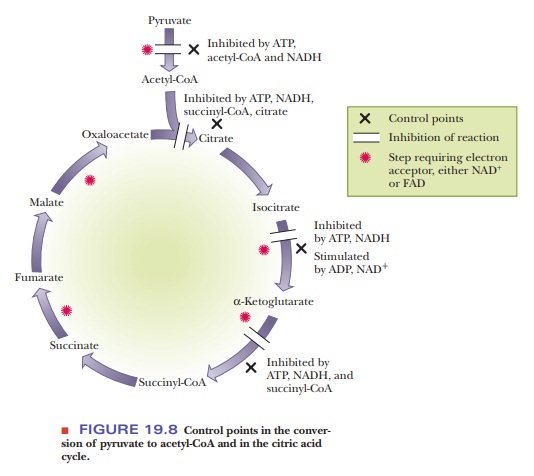
the citric acid cycle is exercised at three points; that is, three enzymes
within thecitric acid cycle play a regulatory role (Figure 19.8). There is also
control of access to the cycle via pyruvate dehydrogenase.
How does the pyruvate dehydrogenase reaction control the citric acid cycle?
The overall reaction is part of a pathway that releases energy. It is not surprising that the enzyme that initiates it is inhibited by ATP and NADH because both compounds are abundant when a cell has a good deal of energy readily available. The end products of a series of reactions inhibit the first reaction of the series, and the intermediate reactions do not take place when their products are not needed. Consistent with this picture, the pyruvate dehydrogenase (PDH) complex is activated by ADP, which is abundant when a cell needs energy.
In mammals, the actual mechanism by which the inhibition takes
place is the phosphorylation of pyruvate dehydrogenase. A phosphate group is
covalently bound to the enzyme in a reaction catalyzed by the enzyme pyruvatedehydrogenase kinase. When the
need arises for pyruvate dehydrogenase to beactivated, the hydrolysis of the
phosphate ester linkage (dephosphorylation) is catalyzed by another enzyme, phosphoprotein phosphatase. This latter
enzyme is itself activated by Ca2+. Both enzymes are associated
with the mammalian pyruvate dehydrogenase complex, permitting effective control
of the overall reaction from pyruvate to acetyl-CoA. The PDH kinase and PDH
phosphatase are found on the same polypeptide chain. High levels of ATP
activate the kinase. Pyruvate dehydrogenase is also inhibited by high levels of
acetyl-CoA. This makes a great deal of metabolic sense. When fats are plentiful
and are being degraded for energy, their product is acetyl-CoA. Thus, if
acetyl-CoA is plentiful, there is no reason to send carbohydrates to the citric
acid cycle. Pyruvate dehydrogenase is inhibited, and the acetyl-CoA for the TCA
cycle comes from other sources.
How is control exerted within the citric acid cycle?
Within
the citric acid cycle itself, the three control points are the reactions
catalyzed by citrate synthase, isocitrate dehydrogenase, and the ╬▒-ketoglutarate dehydrogenase complex. We have already mentioned
that the first reaction of the cycle is one in which regulatory control
appears, as is to be expected in the first reaction of any pathway. Citrate
synthase is an allosteric enzyme inhibited by ATP, NADH, succinyl-CoA, and its
own product, citrate.
The
second regulatory site is the isocitrate dehydrogenase reaction. In this case,
ADP and NAD+ are allosteric activators of the enzyme. We have called
attention to the recurring pattern in which ATP and NADH inhibit enzymes of the
pathway, and ADP and NAD+ activate these enzymes.
The ╬▒-ketoglutarate dehydrogenase complex is the third regulatory site.
As before, ATP and NADH are inhibitors. Succinyl-CoA is also an inhibitor of
this reaction. This recurring theme in metabolism reflects the way in which a
cell can adjust to an active state or to a resting state.
When a cell is metabolically active it uses ATP and NADH at a great rate, producing large amounts of ADP and NAD+ (Table 19.3). In other words, when the ATP/ADP ratio is low, the cell is using energy and needs to release more energy from stored nutrients.
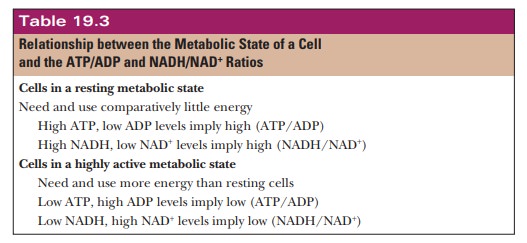
A low NADH/NAD+ ratio is also characteristic
of an active metabolic state. On the other hand, a resting cell has fairly high
levels of ATP and NADH. The ATP/ADP ratio and the NADH/NAD+ ratio
are also high in resting cells, which do not need to maintain a high level of
oxidation to produce energy.
When
cells have low energy requirements (that is, when they have a high ŌĆ£energy
chargeŌĆØ) with high ATP/ADP and NADH/NAD+ ratios, the presence of so
much ATP and NADH serves as a signal to ŌĆ£shut downŌĆØ the enzymes responsible for
oxidative reactions. When cells have a low energy charge, char-acterized by low
ATP/ADP and NADH/NAD+ ratios, the need to release more energy and to
generate more ATP serves as a signal to ŌĆ£turn onŌĆØ the oxidative enzymes. This
relationship of energy requirements to enzyme activity is the basis for the
overall regulatory mechanism exerted at a few key control points in metabolic
pathways.
Summary
The citric acid cycle is exergonic in terms of overall free-energy
changes. In addition, it produces four NADH and one FADH2 for
each pyruvate that enters the cycle. Reoxidation of these electron carriers
produces 25 ATP.
Four control points exist for the citric acid cycle. One, the
pyruvate dehy-drogenase reaction, lies outside the cycle proper. The formation
of citrate and the two oxidative decarboxylations are the other control points.
ATP and NADH are inhibitors of the cycle, and ADP and NAD+ are
activators.
Related Topics