Chapter: Biochemistry: The Citric Acid Cycle
The Citric Acid Cycle in Anabolism
The Citric Acid Cycle in
Anabolism
The citric acid cycle is a source of starting materials for the
biosynthesis of many important biomolecules, but the supply of the starting
materials that are components of the cycle must be replenished if the cycle is
to continue operating.. In particular, the oxaloacetate in an organism must be
maintained at a level sufficient to allow acetyl-CoA to enter the cycle. A
reaction that replenishes a citric acid cycle intermediate is called an anaplerotic reaction. In some
organisms, acetyl-CoA can be converted to oxaloacetate and other citric acid
cycle intermediates by the glyoxylate cycle, but mammals cannot do this. In
mammals, oxaloacetate is produced from pyruvate by the enzyme pyruvate carboxylase (Figure 19.11). We
already encountered this enzyme and this reaction in the context of
gluconeogenesis, and here we have another highly important role for this enzyme
and the reaction it catalyzes. The supply of oxaloacetate would soon be depleted
if there were no means of producing it from a readily available precursor.
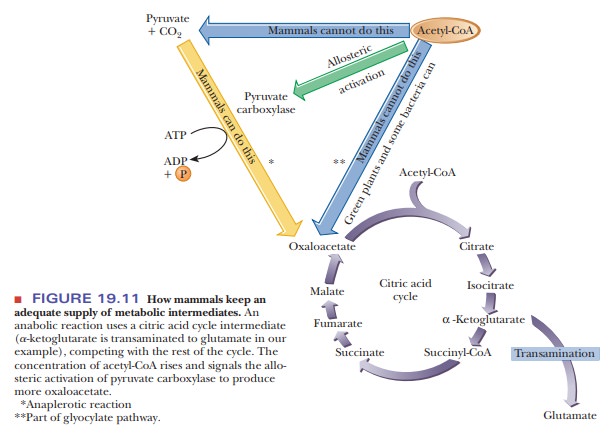
This reaction, which produces oxaloacetate from pyruvate, provides
a con-nection between the amphibolic citric acid cycle and the anabolism of
sugars by gluconeogenesis. On this same topic of carbohydrate anabolism, we
should note again that pyruvate cannot be produced from acetyl-CoA in mammals.
Because acetyl-CoA is the end product of catabolism of fatty acids, we can see
that mammals could not exist with fats or acetate as the sole carbon source.
The intermediates of carbohydrate metabolism would soon be depleted.
Carbohydrates are the principal energy and carbon source in animals (Figure
19.11), and glucose is especially critical in humans because it is the
preferred fuel for our brain cells. Plants can carry out the conversion of
acetyl-CoA to pyruvate and oxaloacetate, so they can exist without
carbohydrates as a carbon source. The conversion of pyruvate to acetyl-CoA does
take place in both plants and animals.
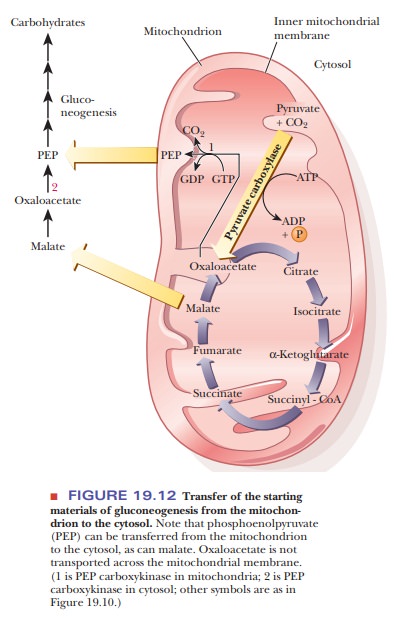
The anabolic reactions of gluconeogenesis take place in the
cytosol. Oxaloacetate is not transported across the mitochondrial membrane. Two
mechanisms exist for the transfer of molecules needed for gluconeogenesis from
mitochondria to the cytosol. One mechanism takes advantage of the fact that
phosphoenolpyruvate can be formed from oxaloacetate in the mitochon-drial
matrix (this reaction is the next step in gluconeogenesis);
phosphoenol-pyruvate is then transferred to the cytosol, where the remaining
reactions take place (Figure 19.12). The other mechanism relies on the fact
that malate, which is another intermediate of the citric acid cycle, can be
transferred to the cytosol. There is a malate
dehydrogenase enzyme in the cytosol as well as in mito-chondria, and malate
can be converted to oxaloacetate in the cytosol.oxaloacetate is produced from
pyruvate by the enzyme pyruvate
carboxylase (Figure 19.11). We already encountered this enzyme and this
reaction in the context of gluconeogenesis, and here we have another highly
important role for this enzyme and the reaction it catalyzes. The supply of
oxaloacetate would soon be depleted if there were no means of producing it from
a readily available precursor.
This reaction, which produces oxaloacetate from pyruvate, provides
a con-nection between the amphibolic citric acid cycle and the anabolism of
sugars by gluconeogenesis. On this same topic of carbohydrate anabolism, we
should note again that pyruvate cannot be produced from acetyl-CoA in mammals.
Because acetyl-CoA is the end product of catabolism of fatty acids, we can see
that mammals could not exist with fats or acetate as the sole carbon source.
The intermediates of carbohydrate metabolism would soon be depleted.
Carbohydrates are the principal energy and carbon source in animals (Figure
19.11), and glucose is especially critical in humans because it is the
preferred fuel for our brain cells. Plants can carry out the conversion of
acetyl-CoA to pyruvate and oxaloacetate, so they can exist without
carbohydrates as a carbon source. The conversion of pyruvate to acetyl-CoA does
take place in both plants and animals.
The anabolic reactions of gluconeogenesis take place in the cytosol. Oxaloacetate is not transported across the mitochondrial membrane. Two mechanisms exist for the transfer of molecules needed for gluconeogenesis from mitochondria to the cytosol. One mechanism takes advantage of the fact that phosphoenolpyruvate can be formed from oxaloacetate in the mitochon-drial matrix (this reaction is the next step in gluconeogenesis); phosphoenol-pyruvate is then transferred to the cytosol, where the remaining reactions take place (Figure 19.12). The other mechanism relies on the fact that malate, which is another intermediate of the citric acid cycle, can be transferred to the cytosol. There is a malate dehydrogenase enzyme in the cytosol as well as in mito-chondria, and malate can be converted to oxaloacetate in the cytosol.
Oxaloacetate is then converted to phosphoenolpyruvate, leading to
the rest of the steps of gluconeogenesis (Figure 19.12).
Gluconeogenesis has many steps in common with the production of
glucose in photosynthesis, but photosynthesis also has many reactions in common
with the pentose phosphate pathway. Thus, nature has evolved common strategies
to deal with carbohydrate metabolism in all its aspects.
How is lipid anabolism related to the citric acid cycle?
The starting point of lipid anabolism is acetyl-CoA. The anabolic
reactions of lipid metabolism, like those of carbohydrate metabolism, take
place in the cytosol; these reactions are catalyzed by soluble enzymes that are
not bound to membranes. Acetyl-CoA is mainly produced in mitochondria, whether
from pyruvate or from the breakdown of fatty acids. An indirect transfer
mechanism exists for transfer of acetyl-CoA in which citrate is transferred to
the cytosol (Figure 19.13). Citrate reacts with CoA-SH to produce citryl-CoA,
which is then cleaved to yield oxaloacetate and acetyl-CoA. The enzyme that
catalyzes this reaction requires ATP and is called ATP-citrate lyase. The
overall reaction is
Citrate + CoA-SH + ATP - > Acetyl-CoA + Oxaloacetate + ADP + Pi
Acetyl-CoA is the starting point for lipid anabolism in both plants
and animals. An important source of acetyl-CoA is the catabolism of
carbohydrates. We have just seen that animals cannot convert lipids to
carbohydrates, but they can convert carbohydrates to lipids. The efficiency of
the conversion of carbohydrates to lipids in animals is a source of
considerable chagrin to many humans.
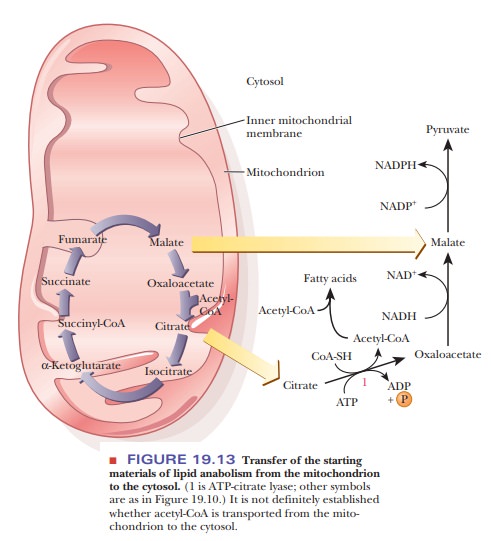
Oxaloacetate can be reduced to malate by the reverse of a reaction.
Oxaloacetate + NADH + H+ ->
Malate + NAD+
Malate can move into and out of mitochondria by active transport
processes, and the malate produced in this reaction can be used again in the
citric acid cycle. However, malate need not be transported back into
mitochondria but can be oxidatively decarboxylated to pyruvate by malic enzyme, which requires NADP+.
Malate + NADP+ -> Pyruvate + CO2 + NADPH
+ H+
These last two reactions are a reduction reaction followed by an
oxidation; there is no net oxidation.
There is, however, a substitution of
NADPH for NADH.
This last point is important because many of the enzymes of fatty
acid synthesis require NADPH. The pentose phosphate pathway is the principal
source of NADPH in most organisms, but here we have another source (Figure
19.14).
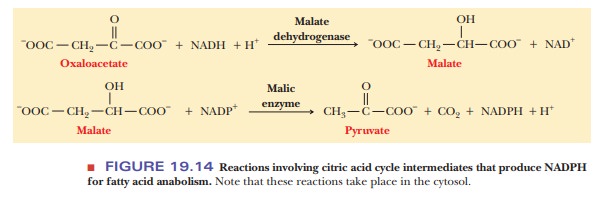
The two ways of producing NADPH clearly indicate that all metabolic
path-ways are related. The exchange reactions involving malate and citryl-CoA
con-stitute a control mechanism in lipid anabolism, while the pentose phosphate
pathway is part of carbohydrate metabolism. Both carbohydrates and lipids are
important energy sources in many organisms, particularly animals.
How is amino acid metabolism related to the citric acid cycle?
The anabolic reactions that produce amino acids have, as a starting point, the intermediates of the citric acid cycle that can cross the mitochondrial membrane into the cytosol.
We have already seen that malate can cross the mitochondrial membrane and give rise to oxaloacetate in the cytosol. Oxaloacetate
can undergo a transamination reaction to produce aspartate, and aspartate, in
turn, can undergo further reactions to form not only amino acids but also other
nitrogen-containing metabolites, such as pyrimidines. Similarly, isocitrate can
cross the mitochondrial membrane and produce α-ketoglutarate in the cytosol. Glutamate arises
from α-ketoglutarate
as a result of another transamination reaction, and glutamate undergoes further
reactions to form still more amino acids. Succinyl-CoA gives rise not to amino
acids but to the porphyrin ring of the heme group. Another difference is that
the first reaction of heme biosynthesis, the condensation of succinyl-CoA and
glycine to form d-aminolevulinic acid (see supplementary material on nitrogen
metabolism on the website), takes place in the mitochondrial matrix, while the
remainder of the pathway occurs in the cytosol.
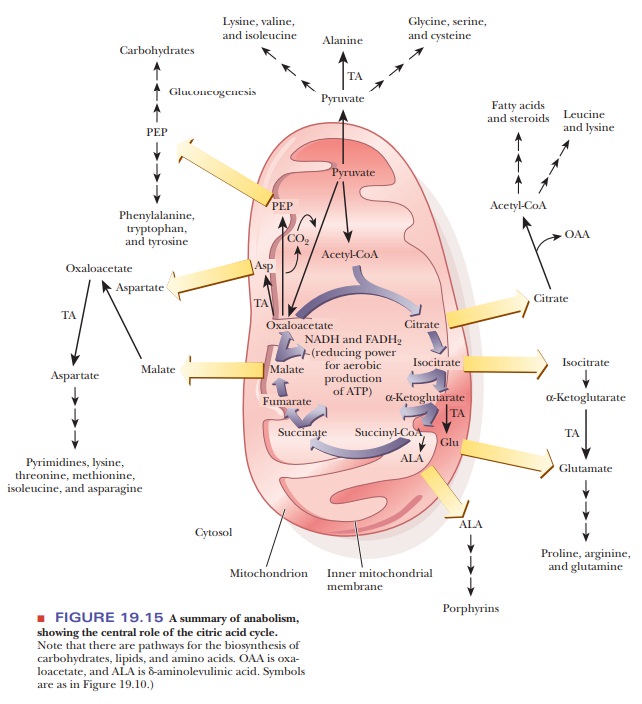
The overall outline of anabolic reactions is shown in Figure 19.15.
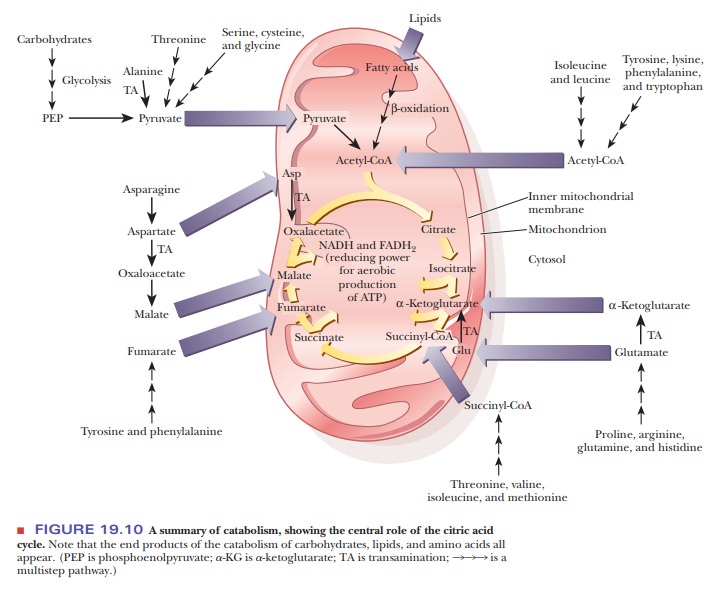
We used the same type of diagram in Figure 19.10 to show the overall outline of
catabo-lism. The similarity of the two schematic diagrams points out that
catabolismand anabolism, while not exactly the same, are closely related. The
operation of any metabolic pathway, anabolic or catabolic, can be “speeded up”
or “slowed down” in response to the needs of an organism by control mechanisms,
such as feedback control. Regulation of metabolism takes place in similar ways
in many different pathways.
Summary
The citric acid cycle plays a central role in anabolic pathways as
well as in catabolism.
Pathways that give rise to sugars, fatty acids, and amino acids all
originate with components of the citric acid cycle.
Related Topics