Chapter: Medical Immunology: Lymphocyte Ontogeny and Membrane Markers
T-Lymphocyte Ontogeny
T-LYMPHOCYTE ONTOGENY
The thymus is the primary organ for T-cell differentiation. When the thymus is congeni-tally absent or surgically removed before immunological maturity is reached, a profound T lymphocyte deficiency is observed. The T-lymphocyte deficiency in athymic and thymec-tomized mice is usually associated with severe immunodeficiency and is incompatible with prolonged survival.
A. Pre–T-Lymphocyte Markers
T lymphocytes differentiate intrathymically from the common lymphoid progenitor origi-nating in the bone marrow as early as the seventh week of gestation. The most primitive thymic immigrants express CD34, CD45RA, and CD7 but do not express some of the adult T-cell markers, such as CD2 and CD3. These cells are tripotential precursors, which have the ability to differentiate into T, NK, or DC cells. The next most primitive cell has the same phenotype, except that it has become CD2+ and CD5+ . The thymocytes then acquire CD1a and are restricted to the T-cell lineage. Expression of CD1a parallels TcR gene rearrange-ments. Although expressed at high levels on thymocytes, CD1 is barely detectable on ma-ture T lymphocytes.
B. Enzymatic Activity in Pre–T Lymphocytes
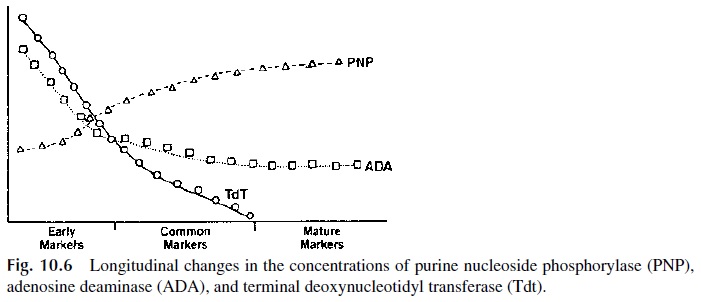
Once they reach the thymus, the T-lymphocyte precursor cells proliferate rapidly. Matur-ing thymocytes express several enzymes of the purine salvage pathway, such as adenosine deaminase (ADA), purine nucleoside phosphorylase (PNP), and terminal deoxynucleotidyl transferase (Tdt). The expression level of these enzymes changes as ontogenic develop-ment proceeds (Fig. 10.6).
C. Rearrangement of the TcR Genes
The TcR genes undergo rearrangements during T-cell differentiation. These rearrange-ments involve the deletion of noncoding sequences and the joining, or transposition, of noncontiguous segments of DNA. Transposition is mediated by the products of the RAG genes, which act as transposases. RAG-deficient mice cannot rearrange B- or T-cell recep-tor genes.
Strategies similar to those used in the studies of the molecular genetics of im- munoglobulin genes allowed the identification of the genes coding for the α, β, γ, and δ chains of the TcR. The β and γ chain genes are located in distant regions of chromosome 7, while the α and δ chain genes are located on chromosome 14 (Fig. 10.7). The rear- rangement of these genes will generate two types of receptors: γδ or TcR1, and αβ or TcR2.
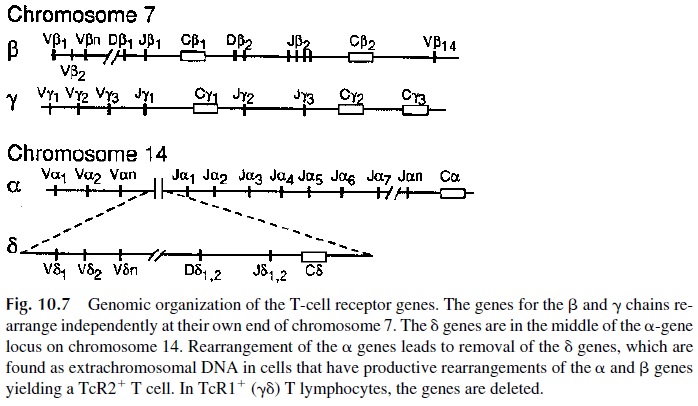
Due to the chromosomal organization of the TcR genes, the TcR1 genes are rear- ranged first. The δ gene is located in the middle of the α-chain gene, between the Vα and Jα regions. When RAG transposes the elements of the δ genes to form a VDJ complex, it eliminates the V and J regions of the α gene. The rearranged δ chain will pair with the γ chain. T cells expressing TcR1 ( γδTcR) will leave the thymus to migrate to the skin and mucosae. Rearrangements of the TcR2 genes occur during the second wave. The appear-ance of a DJ rearrangement of the β chain is followed by the addition of V- and C-region genes. The productively rearranged β chain will be present in the cytoplasm for several days before the α chain is expressed. During that period the, β chain will associate with a “protective” polypeptide, known as the pre-Tα . This pre–T-cell receptor (pre-TcR) is ex-pressed on the surface of T-cell precursors and has signaling capacity.
Rearrangement of the α-chain genes results in the deletion of the δ gene, which will appear as extrachromosomal circular DNA in germline configuration. When the α-chain gene rearrangement is completed, the translated α chain combines with a β chain and a full receptor is expressed on the cell membrane. Coinciding with the synthesis of a complete αβ, the recombinases involved in the processes become inactive, so that rearrangements on the second allele do not take place. Thus, the phenomenon of allelic exclusion, originally described for immunoglobulin synthesis , also exists for TcR synthesis.
The generation of the extensive diversity needed for a complete TcR repertoire relies almost exclusively on the D regions. These regions are the targets of extensive nucleotide addition, particularly at the DJ junction, resulting in configurational diversity.
Of the four genes available for TcR synthesis, only the β and δ genes that have a D region will be hy-pervariable; by contrast, theα and γ genes are less polymorphic. A major difference be-tween T and B cells is that once the rearrangements are completed in the thymus, the TcR will never change again, whereas BcR will undergo class switching and affinity maturation in the secondary lymphoid organs. The estimated diversity of αβ and γδ TcR (1015–1018) exceeds the potential diversity of immunoglobulins (109–1011) by several orders of magni-tude.
D. Association with Coreceptors
Prior to TcR gene rearrangements, early thymocytes do not express CD4 or CD8 and are known as double negative thymocytes. Following productive β-chain rearrangement, β the chain/pre-Tα (pre-TcR) associates with CD3 molecules and is transported to the cell mem-brane. This event generates activation signals, which lead to the co-expression of CD4 and CD8. These cells are then known as double positive thymocytes. As the cell continues to differentiate, one of these two markers will be retained and the other will be lost, so that the cells will be either CD4+ or CD8+ .,
CD3 is a complex of 5 unique subunits designated α, δ, e, ζ, and μ (note that the γ and δ chains of the CD3 unit are distinct from the γδ chains of the TcR1). The CD3 γδe trimolecular complex is synthesized first and remains intracytoplasmic, where it becomes associated with pre-Tα molecules. Soon thereafter, the CD3 ζ chains are synthesized and become associated to the CD3 complex. Once the ζ chain has been added to the CD3 com-plex, the whole CD3–pre-Tα complex is transported from the Golgi apparatus to the cell membrane. A critical characteristic of the ζ chain is its long intracytoplasmic tail, which has affinity for the zeta-associated protein kinase (ZAP70). The association of ZAP70 to the ζ chain is critical for further differentiation of the T cell. The congenital absence of ZAP70 is associated with a block at the DN stage of T-cell development.
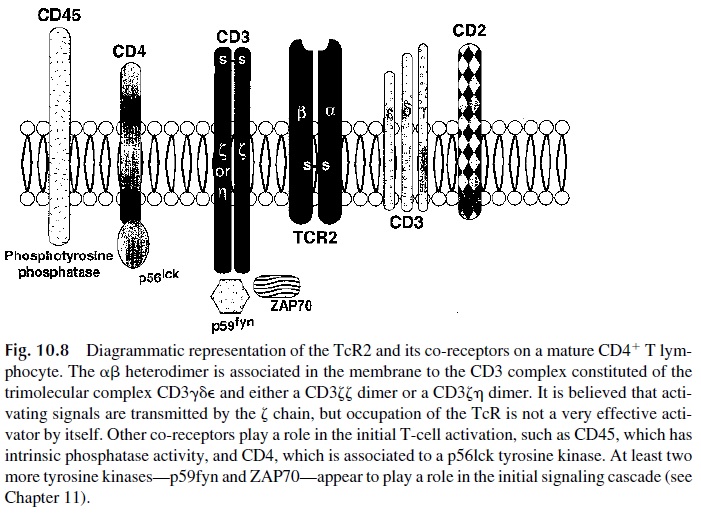
Once the CD3-pre-Tα complex is expressed on the cell membrane, RAG genes are reexpressed. Subsequently, α-gene rearrangements occur, followed by the replacement of the pre-Tα chain of the pre–T-cell receptor by the newly synthesized α chain. The αβ complex, with a full complement of CD3 molecules, is then inserted in the T-cell membrane (Fig. 10.8).
E. Selecting the Right Receptors
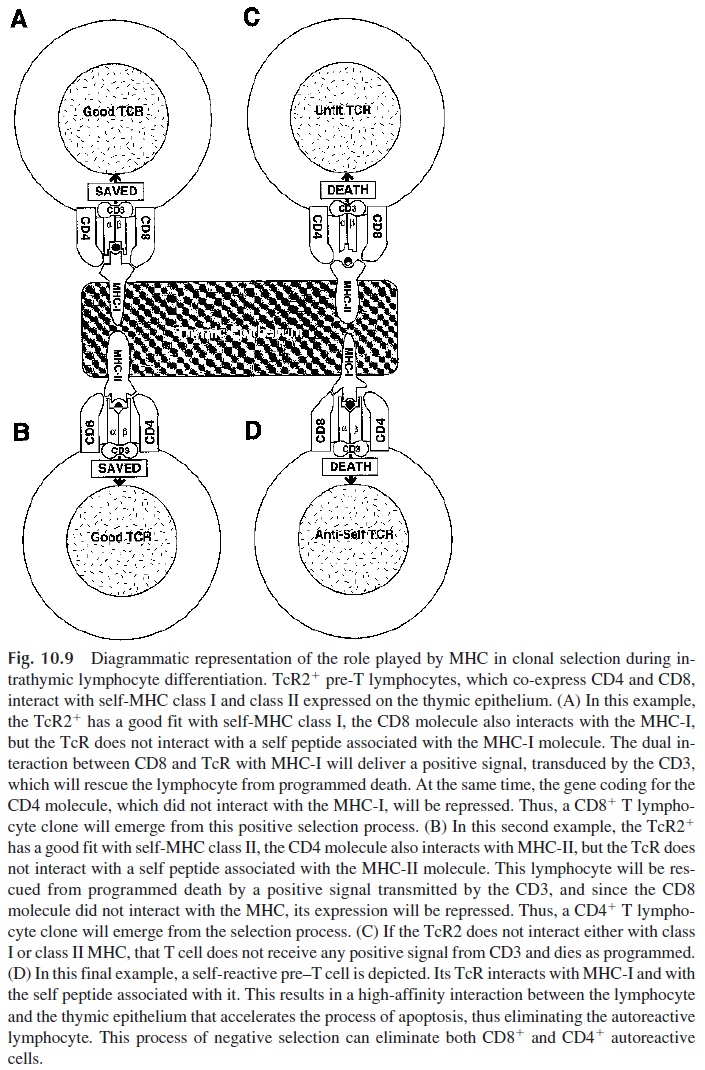
Positive and negative selection of differentiating T cells also occurs in the thymus. Coin-ciding with the expression of complete TcR, double-positive pre–T cells move from corti-cal area of the thymus to the medullary area. Thymic epithelial cells in the medulla, which express both MHC class I and class II, interact with the pre-TcR cells. At this time, au-toreactive and useless T cells are eliminated (Fig. 10.9). Only 2–3% of the differentiating T cells survive this process.
1. Negative Selection
Many newly formed pre–T cells are negatively selected because the TcR is abnormal (the recombination process produced out of frame rearrangements) or because the resulting TcR is unable to interact with any HLA molecule. A smaller percentage is thought to die from apoptosis because their interaction with the HLA peptide complexes is too strong.
This last mechanism is a consequence of the fact that during the time T cells are differentiating, ex-tensive development of most other tissues is taking place, hence, large numbers of cells are undergoing apoptotic death. This results in the extensive release of self antigens, which are eventually captured by the thymic epithelial cells and presented to the pre–T cells in the context of self MHC. The pre–T cells whose TcR interacts strongly with a MHC-peptide complex receive an apoptotic signal and are eliminated. This negative selection process in responsible, in part, for the development of tolerance to self during embryonic differentia-tion .
2. Positive Selection
The few double positive pre–T cells that survive are those that have TcR molecules able to interact at the same time with MHC molecules and with either the CD4 or the CD8 molecule. If the TcR is MHC class I restricted, it must associate with CD8. The CD8-TcR MHC class I interaction results in the activation of repressor genes that turn off CD4 ex-pression. Conversely, double positive T cells that have MHC class II restricted TcR engage CD4 and the expression of CD8 is turned off. The single positive CD4 or CD8 T cells emerging from the medulla leave the thymus and colonize the peripheral lymphoid organs.
The positive signal delivered to the double positive thymocytes through these TcR-MHC interactions involves CD45 and the lymphocyte-specific tyrosine kinase p561ck. These molecules are utilized by both CD4 and CD8 T cells. As the TcR interacts with MHC, the phosphatase activity of CD45 is upregulated and ZAP70 is activated. Both ac-tivities contribute to the dephosphorylation of p56lck, which becomes activated. The acti-vation of p561ck is critical for further T-cell differentiation, because mice deficient in either CD45 or p56lck cannot proceed beyond the double positive stage.
Also critical is the proper expression of MHC molecules: MHC class I–deficient mice do not develop normal numbers of CD8+ T cells, while class II–deficient mice and humans do not differentiate normal numbers of CD4+ T cells.
F. T-Cell Differentiation After Birth
The thymus remains active as the site of T-cell differentiation until early adulthood. Later in adult life the thymus becomes atrophic. An adequate supply of T cells may be due to the fact that memory T cells survive for decades. Alternatively, extrathymic differentiation of T cells may occur in adult life.
γδ T cells expressing TcR1 abound in the mucosal immune system and the skin, where they represent the dominant T-cell population. The precise biological role of TcR1+ T lymphocytes is a matter of considerable debate. The αβ T cells, expressing TcR2, repre-sent more than 95% of mature circulating T lymphocytes.
G. TcR Structure
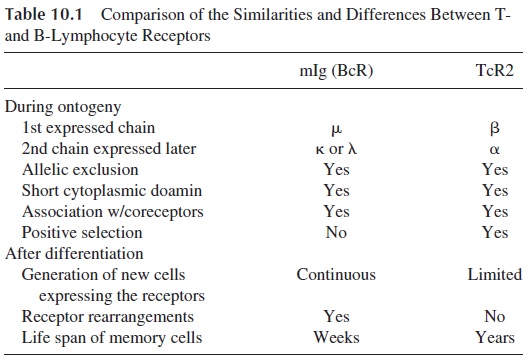
Although the structure of TcR2 (αβ ) has been more extensively characterized than the structure of TcR1 (γδ ), both heterodimers are believed to have a similar structure. The com-parison of the general characteristics of TcR and surface immunoglobulin in B lympho-cytes (BcR) reveals many similarities, as well as notable differences. A comparison be-tween the two is summarized in Table 10.1
The α (acidic) chain is about 40–50 kDa, whereas, the β (basic) chain is slightly smaller, 40–45 kDa. Each chain is comprised of an extracellular, a transmembrane, and an intracytoplasmic domain. Like the BcR, the TcR has a small intracytoplasmic region. In the extracellular domain, the constant region contains about 120–140 amino acids and the vari-able domains 100–120 amino acids. The variable regions are located on the amino-termi-nal end of each chain. Their length is similar to that of the immunoglobulin variable regions.
The hypervariable regions within the variable domain form the antigen-binding site, unique for each TcR. Monoclonal antibodies generated against the antigen-binding site of the TcR detect an idiotypic specificity unique to that particular TcR or T-cell clone.
The surface area of the antigen-binding site on the TcR2 is similar to that of the BcR, yet the degree of genetic diversity for TcR2 is more extensive.
The crystallographic structure of an HLA A2 molecule complexed to a viral peptide interacting with a specific T-cell receptor has been examined. The TcR is positioned in di-agonal orientation over the face of the peptide-MHC complex, two loops (CDR1 and CDR2) of the TcR α chain overlie the N terminus of the peptide, and the corresponding area of the β chain interacts with the peptide C terminus. At the same time, both TcR α–chain loops also interact with the MHC molecule. The most variable region of both α and β chains, designated as CDR3, is in the center of the molecule and has the best access to the most variable region of the MHC-associated peptide. It is likely that the same structural re-lationships are operative in the interaction between TcR and MHC class II–peptide complexes.
H. Membrane Markers on Mature T Cells
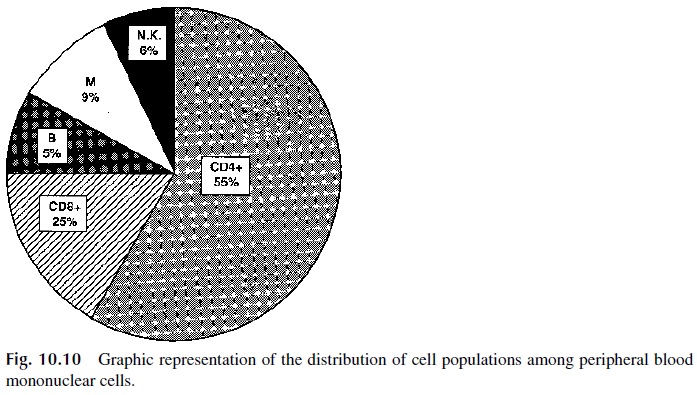
The list of membrane markers present on T cells (and other hematopoietic cells) continues to grow. Generally, markers can be divided into two categories: pan-specific or subset-spe-cific. Pan-specific markers are common to all cells of a given type, whereas subset-specific markers are present on only a subpopulation of cells of a given lineage. CD2 and CD3 are considered to be pan-T-cell markers; however, their specificity is not absolute because CD2 is also expressed by NK cells in general and CD3 is expressed by a special subpopulation of NK cells known as T/NK (CD3), highlighting the lineage relationship between T and NK cells. CD4 and CD8 represent subset-specific T-cell markers. As shown in Figure 10.10, about three quarters of peripheral blood mononuclear cells are T lymphocytes, and among T lymphocytes CD4+ cells predominate over CD8+ cells by a 2:1 ratio.
CD3 is first expressed during thymic differentiation and continues to be expressed all mature T cells in the periphery. CD3 is a complex of five unique, invariant chains (γδeζη ). The cytoplasmic domains of the CD3ζ chain contain ITAM motifs and are responsible for signaling. The x chain also associates with CD16 and functions as the signaling domain of that receptor expressed on NK cells. Phosphorylated ITAM motifs of the CD3ζ chain bind to SH2 domains of the intracellular signaling molecules, e.g., phosphorylated ζ chains bind ZAP70.
CD2 is expressed on all thymocytes, T cells, and NK cells. The heterotypic interac-tion between CD2 and its major ligand CD58 (LFA-3) enhances T-cell antigen recognition. CD2:CD58 interaction can also stimulate T-cell proliferation and differentiation (see Chap-ter 11).
CD4 is expressed on most thymocytes and approximately two thirds of the peripheral blood T cells. CD4 is also expressed on monocytes and macrophages. CD4 defines the T-helper cell population and binds to MHC class II antigens during antigen-specific TcR binding. CD4 is also the receptor for HIV-1.
CD8 is expressed on most thymocytes, a third of the peripheral blood T cells, and some NK cells. CD8 can be expressed as a CD8αβ heterodimer or CD8αα homodimer. CD8αβ is only found on TcR2 (αβ) T cells. In contrast, CD8αα can be found on NK cells,T γδ cells, or αβ T cells. CD8αβ T cells represent the cytotoxic subpopulation of T lym- phocytes. CD8 binds to MHC class I antigens during antigen-specific TcR binding. The function of CD8 on NK cells is not known.
1. Markers Associated with T-Cell Activation
CD25, the low-affinity IL-2 receptor (IL-2R), is expressed at low levels by about 30% of circulating (resting) lymphocytes, particularly on the memory T-cell population that ex-presses CD45RO or CD45RA and is CD62L- . Expression is upregulated upon T-cell acti-vation; thus, CD25 expression can be used as an indicator of the number of activated T cells. Activated B lymphocytes also express CD25.
CD2R is an epitope of CD2 expressed by activated T lymphocytes and is probably the most specific of the activation markers. Its expression is likely to be associated with cell-cell interactions involving CD2 and LFA-3, which are believed to deliver additional activation signals to the T lymphocyte.
CD45 expression and function has been characterized more extensively in T lym-phocytes than in other leukocyte populations. CD45 plays an important role in T-lympho-cyte activation, due to the fact that its intracytoplasmic domain has tyrosine phosphatase activity. Three isoforms (CD45RA, CD45RB, CD45RO) have been identified on T cells. These isoforms share the intracellular domains but vary in their extracellular domains. CD45RA is the largest (220 kDa), CD45RB is of intermediate size (205 kDa), and CD45RO the smallest (180 kDa). The ontogenic development of the CD45 isoforms is not clear, but in mature lymphocytes their expression becomes restricted (as indicated by the letter R in the designation of these isoforms).
CD45RA is expressed both by naive and memory CD4+ T cells. In the case of naive populations, CD45 is co-expressed with CD62L, while memory T cells either express CD45RA only or switch from CD45RA to CD45RO.
CD45RO+ T cells are considered as either as primed T lymphocytes or as memory T lymphocytes (the expression of this marker seems to be maintained long after the primary response has waned). CD45RO+, CD4+ T cells are thought to provide B-cell help during humoral immune responses.
CD28 is a disulfide-linked homodimer expressed on most T-lineage cells and plasma cells. All CD4+ cells and half of the CD8+ circulating lymphocytes express CD28. The dominant costimulatory pathway in T-cell immune responses results from the interaction of CD28 and a related molecule, CD152 (CTLA-4), with the B-lymphocyte antigens CD80 (B7.1) and CD86 (B7.2) .
CD71, the transferrin receptor, is a type II membrane glycoprotein that is upregulated during leukocyte activation. The CD71 homodimer associates with CD3 ζ, suggesting a role in signal transduction. The CD71-controlled supply of iron to the cell is important during proliferative responses.
CD69 is a lectin receptor encoded in a region of chromosome 12 known as the “NK gene complex.” CD69 is expressed on a variety of hematopoietic cells. Although absent on resting lymphocytes, CD69 is rapidly upregulated during activation of T, B, and NK cells. Because monoclonal antibodies directed against CD69 can activate lymphocytes, a role for CD69 in signal transduction has been suggested.
CD49b, an 2 integrin, is the subunit of VLA-2; the subunit is the integrin CD29. CD49b is expressed on monocytes, platelets, T, B, and NK cells. It is upregulated upon stimulation with phytohemagglutinin. VLA-2 (CD49b/CD29) binds collagen and laminin.
CD154 [CD40 ligand (CD40L)] is expressed on activated CD4+ cells and a small population of CD8+ cells and γδ T cells. CD154 interacts with CD40, expressed on B cells. This interaction is required for the development and maturation of T-dependent B-lym-phocyte responses. The lack of expression of the CD154 is the molecular basis of most cases of a unique immunodeficiency disease known as hyper-IgM syndrome.
CD26 is expressed by both hematopoietic and nonhematopoietic cell types. In leuko-cytes, it is upregulated during T-cell activation but primarily expressed on memory T cells. CD26 is a type II membrane dipeptidylpeptidase. CD26 has many proposed functions, mainly that of a membrane-bound protease that facilitates diapedesis and extravascular mi-gration. In addition, there is evidence suggesting that CD26 may function as a cell adhesion molecule and as a T-cell co-stimulatory molecule.
CD54 or ICAM-I is expressed by both hematopoietic and nonhematopoietic cell types. The expression on leukocytes is upregulated during activation. Similarly, the ex-pression on the endothelium is upregulated during inflammation. ICAM-1, ICAM-2 (CD102), and ICAM-3 (CD53) are all coreceptors for the integrin LFA-1 (CD11a/CD18) expressed on antigen-presenting cells (APC) and other leukocytes. ICAM-1 also binds to other related integrins such as CD11b/CD18 (MAC-1) and CD11c/CD18 (p150, 95). On T cells CD54 binding increases antigen-specific interactions with the APC.
2.Markers That Inhibit T-Cell Activation
Related Topics