Chapter: Medical Immunology: Lymphocyte Ontogeny and Membrane Markers
B Lymphocyte Ontogeny
B LYMPHOCYTE ONTOGENY
B lymphocytes are identified by the presence of surface immunoglobulin (slg). After anti-genic stimulation, B cells differentiate into plasma cells that secrete large quantities of immunoglobulins. Within a single cell or a clone of identical cells, the antibody-binding sites of membrane and secreted immunoglobulins are identical. Many steps are involved in the genetic control of immunoglobulin synthesis and the generation of binding site diversity (Fig. 10.3).
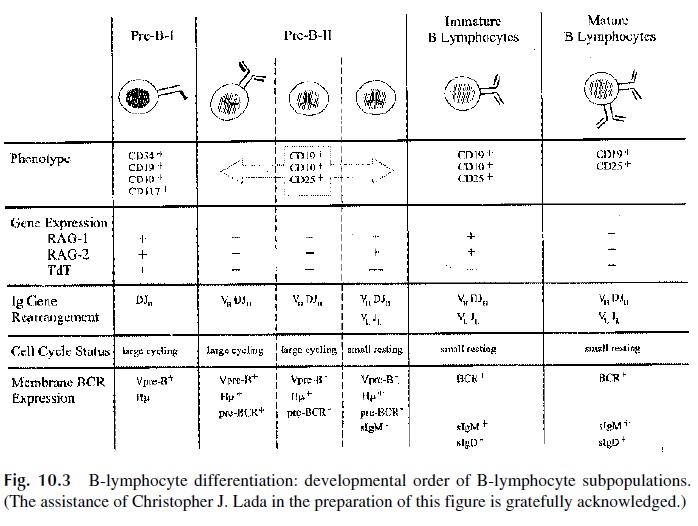
A. Developmental Order of B-Lineage Subpopulations
As B-cell differentiation proceeds, discrete populations of B-lymphocyte precursor cells can be identified based on (1) the expression of specific gene products on the cell mem-brane or in the cytoplasm, in particular, recombinase-activating genes (RAG1, RAG2) and terminal deoxynucleotidyl transferase (Tdt) (2) the status of immunoglobulin gene rear-rangement, (3) the cell cycle status and expression of pre–B-cell receptor and BcR com-plexes. For the most part, B-cell development in the mouse and the human follow similar pathways.
Pre–B-I cells in the human bone marrow express CD34, CD19, CD10, and CD117 (stem cell growth factor receptor) on the membrane and express Tdt, RAG1, and RAG2 in the nucleus. These cells do not express slg or markers present on fully differentiated B cells, such as CD25. Rearrangement of the V-region gene segments has been initiated, but the im-munoglobulin heavy chain (μ chain) is not yet expressed in the cytoplasm. A surrogate light chain formed by the association of the Vpre-B and l5 gene products, alone or in as-sociation with a surrogate chain, is expressed on the cell surface.
Pre–B-II cells downregulate CD117 and Tdt and upregulate CD25. Productive VHDJH rearrangement results in high levels of cytoplasmic μ chains that may associate with surrogate light chain to form a pre–B receptor. A second wave of RAG1 and RAG2 expression is associated with rearrangement of the immunoglobulin light chain (VLJL) and in the resulting synthesis of kappa (k ) or lambda (l) light chains. The majority of the pre–B-II cells are of the small phenotype (VLJL-rearranged, slg+ ). In the mouse and the human, about 80% of the slg+ , CD19+ cells in the bone marrow are pre–B-II cells.
The physiological role of surrogate light chain seems to be that of a stabilizer, pro-tecting nascent μ chains from degradation until light chain synthesis is turned on. The sig-nificance of this phenomenon in B-cell development has yet to be defined, but expression of Vpre-B and l5 genes is critical to normal B-cell development. The l5 gene knockout mice do not form a pre-BcR and are B-cell deficient. In cases of human common variable immunodeficiency associated with defective λ5 genes, the B-cell deficiency is even more pronounced than that observed in the knockout mouse. The absence of the RAG gene prod-ucts is also associated with a differentiation block but in this case affects both T and B cells and results in severe combined immunodeficiency .
Immature B cells are characterized as resting cells (not cycling), which express CD19, CD10, sIgM, and CD79 α/β ( α,β chains of the BcR) but are sIgD- . RAG2 expression is as high in the immature B cell as in the small pre–B-II cell, indicating that additional light-chain gene rearrangement can still occur. Final differentiation occurs when RAG ex-pression is terminated. The phenotype CD19+, CD10- sIgM+ , sIgD+ typifies the mature B cell.
The relative proportion of precursor B cells in the bone marrow remains constant though out the life span of the organism. Pre–B-I cells comprise about 5–10% of the total. Pre–B-II cells represent 60–70%, while the remaining 20–25% are immature B cells.
B. B-Cell Maturation
1. Isotype Switching
Around birth, mature, resting B cells co-express sIgM and sIgD on their membranes. The sIgM and sIgD expressed on individual B-cell clones have the same antigenic specificity. These “virgin” B lymphocytes home to secondary lymphoid organs where, upon anti-genic challenge, they downmodulate sIgD and, less constantly, sIgM. Activated B cells undergo subsequent heavy-chain constant region gene rearrangements or isotype switch-ing. Thus the same variable region is now associated with a different heavy-chain isotype (IgG, IgE, or IgA). The resultant sIg of different isotypes are expressed on nonoverlap-ping B-cell subsets, sometimes in association with sIgM. In addition to isotype switch-ing, activated B cells undergo antibody affinity maturation, which results in the emer-gence and selection of B-cell clones producing antibodies of similar specificity but higher affinity .
2. Ontogenic Development of Immunoglobulin Synthesis
A normal newborn infant, though having differentiated B lymphocytes, produces very small amounts of immunoglobulins for the first two to three months of life. During that pe-riod of time, the newborn is protected by placentally-transferred maternal IgG, which starts to cross the placenta at the 12th week of gestation. By the 3rd month of age, IgM antibod-ies produced by the newborn are usually detectable. The concentration of circulating IgM reaches adult levels by 1 year of age. It must be noted, however, that in cases of intra-uter-ine infection, IgM antibodies are synthesized in relatively large amounts by the fetus and detected in cord blood by conventional assays. The onset of the synthesis of IgG and IgA occurs later and the concentration of these reaches adult levels at 6 to 7 years of age.
C. Surface Molecules Involved in B-Cell Activation and Regulation
Many molecules have been identified on the membrane of B cells. Most are involved in cell-to-cell interactions and participate in the delivery of activating signals or inhibitory signals to the cells (Fig. 10.4). Each B-cell receptor includes molecules responsible for pos-itive signaling—Ig α(CD79α), Igβ (CD79β ), and CD19—as well as downregulatory molecules—CD22, a tyrosine kinase known as lyn and CD32 (FcγRII). A subtle balance between their relative strength is essential for the maintenance of homeostasis after the re-sponse, but the details about their control in vivo remain unclear.
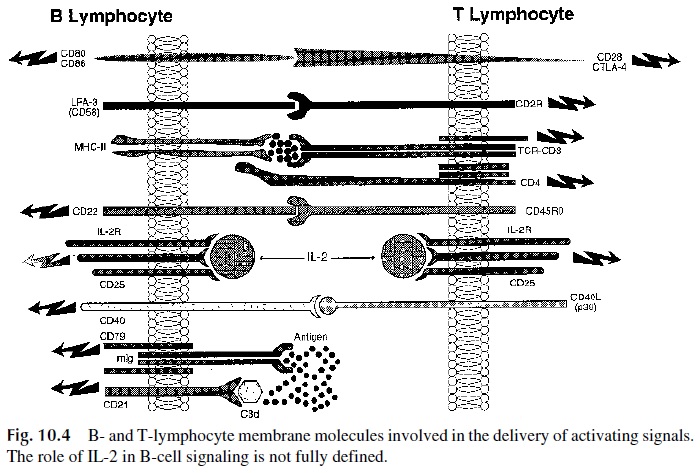
1. Surface Molecules Involved in B-Cell Activation
BcR is formed by the covalent association of sIg with the above-mentioned signaling pro-teins, Igα and Igβ (Fig. 10.5). Antigen recognition occurs though the sIg moiety of the BcR, whereas Ig and Ig mediate signal transduction. After antigen binding, the recruitment of
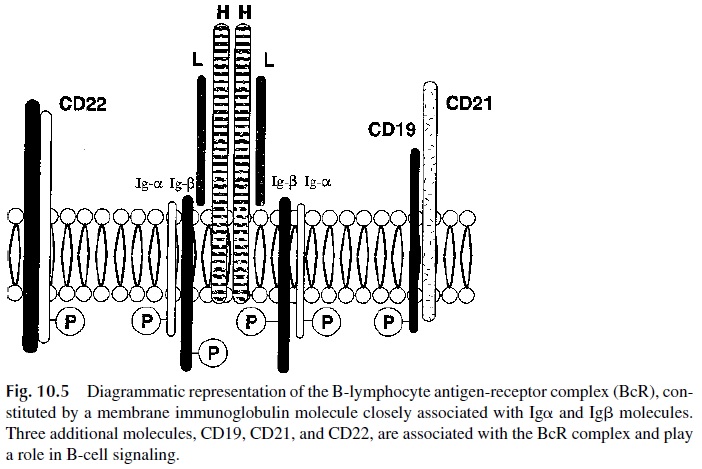
The major kinase that is recruited to the BcR complex is known as Bruton’s tyrosine kinase (Btk). Congenital Btk deficiency is associated with a block in B-cell dif-ferentiation, demonstrating that signals delivered through the fully assembled BcR and as-sociated kinases are necessary for B-cell development during ontogeny.
CD19 is expressed on B-cell lineage cells with the exception of plasma cells. It is also found on most malignancies of B-lymphocyte origin and follicular den-dritic cells. CD19 contains potential phosphorylation sites in the cytoplasmic domain, mod-ulates Ca2+ influx into the cell, and associates with CD21 and other molecules. The for-mation of this molecular complex lowers the activation threshold for the BcR.
CD21 is expressed with high density on mature, resting B cells but is lost upon acti-vation. CD21 is also known as CR2, because this molecule functions as a receptor for the iC3b and C3d fragments of complement . CD21 is also the receptor used by Epstein-Barr virus to infect B lymphocytes, its only target cell.
The cytoplasmic domain of CD21 contains potential phosphorylation sites. In the right circumstances the interaction between antigen-bound C3d and CD21 can deliver a co-stimulating signal to the B cell, which results in significant amplification of the humoral immune response. Mice deficient in CD21 show an impaired response to T-dependent anti-gens. Moreover, CD21 and CD23 (Fc RII) interact on the cell surface, and this interaction may play a regulatory role in IgE production.
CD20 is an antigenic cluster associated with the first membrane marker to be found on a developing B lymphocyte, originally designated as B1. It is detectable on pre–B lym-phocytes expressing cytoplasmicµ chains and remains expressed during maturation on the mature B lymphocyte, but is not expressed on plasma cells. It is an unusual molecule in that it crosses the membrane several times, has only 42 amino acid residues exposed on the out-side, and both the amino and the carboxyl terminal ends are in the cytoplasm. The carboxyl terminal end has 15 serine and threonine residues, the hallmark of a protein susceptible to phosphorylation by protein kinases, which occurs after mitogenic stimulation. This sug-gests that CD20 may play an important role in the activation and proliferation of mature B lymphocytes .
CD45, initially known as the leukocyte common antigen (LCA), is expressed by all leukocytes and their precursors. It is a major cell surface component of normal leukocytes where it occupies up to 10% of the surface and exists in multiple isoforms generated by al-ternate splicing of nuclear RNA. B lymphocytes express only the highest molecular weight isoform of CD45. The most remarkable feature of CD45 is its cytoplasmic domain which comprises 705 amino acids and is the largest intracytoplasmic domain of all known mem-brane proteins. This intracytoplasmic domain has intrinsic tyrosine phosphatase activity and plays an essential role in lymphocyte activation.
CD38 is expressed on immature B and T cells, activated T cells, and terminally dif-ferentiated B cells, but not on resting lymphocytes. CD38 disappears from the membrane of memory B cells differentiated in the mantle zone of the lymph nodes, which subse-quently leave the nodes as CD20+ , CD38- memory B cells and migrate to different lym-phoid organs. Antibodies to CD38 induce T- and B-cell proliferation.
CD58 (leukocyte function–associated 3, LFA3) is expressed on most hematopoietic cells including erythrocytes, as well as various nonhematopoietic cells such as fibroblasts and endothelial and epithelial cells. It is found on about half of the circulating T and B cells. In lymphoid tissues CD58 is expressed on all dendritic cells, macrophages, germinal cen-ter B cells, medullary thymocytes, and medullary thymic epithelial cells. Expression is high on monocytes, memory T cells, and dendritic cells. CD58 on antigen-presenting cells (APC) binds to its coreceptor, CD2, on T cells. This interaction results in increased inter-cell adhesion and in the delivery of co-stimulatory signals to the T cell.
CD80 (B7.1) and CD86 (B7.2) are membrane glycoproteins expressed at low levels on resting B cells and other APC, which are upregulated upon activation. They bind CD28 and CTLA-4, expressed on T lymphocytes .
CD40 interacts with CD154, also known as CD40 ligand (CD40L) or gp39. CD40 is expressed on all mature B cells but is absent from plasma cells. It is also present on some epithelial, endothelial, DC, and activated monocytes. The interaction of CD40 and its core-ceptor expressed on helper T cells is required for B-lymphocyte maturation and isotype switching.
2. Inhibitory or Downregulatory Molecules on B Cells
CD22, an integral part of the B-cell receptor complex, is first detected in the cytoplasm of pre–B-II cells containing cytoplasmic μ chains. Later it is found on the surface of 75% of sIgM+ immature B cells and on 90% of sIgM+ , sIgD+ mature, resting cells. In the adult, CD22 is expressed at relatively high levels in tissue B cells (e.g., in the tonsils and lymph nodes), but not in circulating B cells. CD22 is upregulated during activation, but is lost in the terminally differentiated plasma cells. CD22 binds to sialylated carbohydrate ligands (e.g., CD45RO) and plays an important regulatory role in B-cell activation by raising the B-cell activation threshold. CD22-deficient mice produce excessive antibody response to antigen stimulation, as well as increased levels of auto-antibodies. In contrast, cross-link-ing of CD22 suppresses the response of B cells to antigenic stimulation because the intra-cytoplasmic segment has numerous immunoreceptor tyrosine-based inhibitory motifs (ITIM) that function as docking sites for a tyrosine phosphatase known as SHP-1. The bind- ing of SHP-1 to CD22 prevents phosphorylation of the kinases needed for further B-cell ac-tivation. In this way, CD19 and CD22 cross-regulate each other because activation through CD22 inhibits the CD19 pathway.
CD32 (FcγRII) is expressed on a range of leukocytes, including monocytes, macrophages, Langerhans cells, granulocytes, B cells, and platelets. CD32, one of two low-affinity IgG Fc receptors, only binds aggregated IgG. The cytoplasmic domains associate with SHP-1 and other downregulatory kinases. Co-ligation of CD32 with membrane Ig (a situation that emerges during the immune response due to the formation of antigen-anti-body complexes in antigen excess, leads to the biding and ac-tivation of SHIP. This is followed by inhibition of inositol-1,4,5-triphosphate (IP3), thus blocking the activation pathways activated after BcR occupation.
3. Other B-Cell Membrane Markers
CD10, also known as cALLA (common acute lymphoblastic leukemia antigen), is ex-pressed on precursor and immature B cells, pre–T cells, neutrophils, and bone marrow stro-mal cells. CD10 is a commonly used marker for pre–B acute lymphocytic leukemias and some lymphomas. This molecule is a member of the type II membrane metalloproteinases and has neutral endopeptidase activity. CD10 knockout mice exhibit enhanced lethality to endotoxin, suggesting a role for CD10 in septic shock modulation. CD10 on bone marrow stromal cells appears to regulate B-cell development, since inhibition of CD10 in vivo en-hances B-cell maturation.
CD5 is expressed on most T lymphocytes and on a small subpopulation of B lym-phocytes. CD5 is found on most chronic lymphocytic leukemias. In nonleukemic individ-uals, B lymphocytes expressing CD5 appear to be activated and committed to the synthe-sis of IgM auto-antibodies. However, there is no apparent correlation between disease activity and the numbers of circulating CD5+ cells; thus, the precise role of these cells re-mains speculative. The cytoplasmic domain of CD5 contains the immunoreceptor tyrosine-based activation (ITAM) motifs and is phosphorylated during T-cell activation. CD5 is thought to be involved in thymic selection and in T-B cell recognition.
CD11a combines with another integrin, CD18, to form leukocyte function–associ-ated molecule 1 (LFA-1). The three ligands for LFA-1 are CD54 (intercellular adhesion molecule-1, ICAM-1), CD102 (ICAM-2) and CD50 (ICAM-3). LFA-1 and the ICAM are expressed by T, B, and NK cells and are responsible for homotypic interactions: The LFA-1 expressed on the T cell can interact with its counterreceptor, ICAM-1, on the B cell and vice versa.
Major histocompatibility complex (MHC) antigens in humans are referred to as the human leukocyte antigen or HLA. B lymphocytes express high levels of both HLA classes I and II. The presence of HLA class II enables B lymphocytes to serve as antigen-present-ing cells. Thus, B lymphocytes are unique in that they have both antigen-specific effector properties (i.e., antibody synthesis) as well as antigen-presenting capabilities.
D. Elimination or Downregulation of Autoreactive B Cells
The membrane immunoglobulins of B-cell precursors include those able to combine with epitopes expressed on self antigens. These autoreac-tive cells are either deleted or rendered nonreactive by means of the inactivation of the sig-nal transduction part of their B-cell receptor.
Related Topics