Chapter: Modern Pharmacology with Clinical Applications: Adrenocortical Hormones and Drugs Affecting the Adrenal Cortex
Steroid Physiology
STEROID
PHYSIOLOGY
Anatomy of the Adrenal Cortex
The mammalian adrenal cortex
is divided into three concentric zones: the zona glomerulosa, zona
fascicu-lata, and zona reticularis. The zona glomerulosa pro-duces hormones,
such as aldosterone, that are responsible for regulating salt and water
metabolism; the zona fasciculata produces glucocorticoids; and the zona
retic-ularis produces adrenal androgens. While secretion by the two inner zones
is controlled by pituitary adreno-corticotropic hormone (corticotrophin, ACTH),
aldos-terone produced by the zona glomerulosa is principally controlled by the
renin–angiotensin system. Desoxy-corticosterone, a mineralocorticoid produced
in the zona fasciculata, is under corticotrophin control.
Steroid Biosynthesis
Although the adrenal cortex
is primarily involved in the synthesis and secretion of corticosteroids, it is
also ca-pable of producing and secreting such steroid interme-diates as
progesterone, androgens, and estrogens. The adrenal gland synthesizes steroids
from cholesterol, which is derived from plasma lipoproteins via the low-and
high-density lipoprotein pathways. Additionally, cholesterol is enzymatically
released extramitochondri-ally from cholesterol esters catalyzed by a
cholesterol ester hydrolase. The corticotrophin-dependent stimula-tion of
cholesterol ester hydrolase activity provides an additional source of
cholesterol for steroidogenesis.
Cholesterol is transported
into the mitochondria of steroidogenic tissue, where side chain cleavage is
car-ried out. In common with other mixed-function oxidase systems, the
cholesterol side chain cleavage requires re-duced nicotinamide-adenine
dinucleotide phosphate (NADPH), oxygen, and a specific cytochrome P450. The rate-limiting
step in steroid biosynthesis is the conversion of cholesterol to pregnenolone (Fig.
60.1).
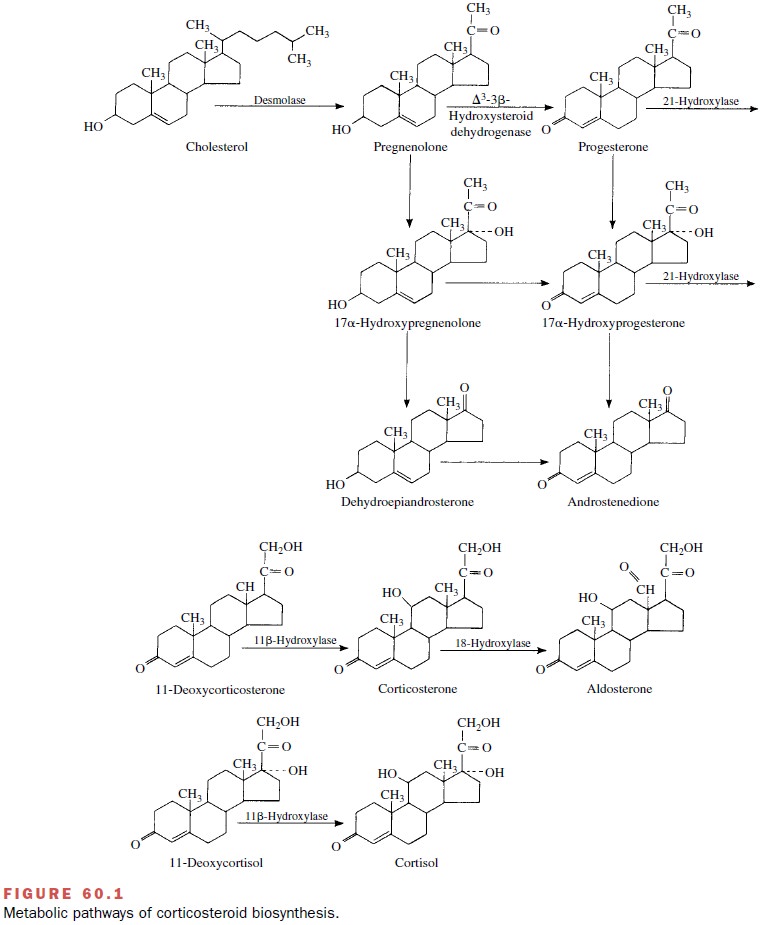
Pregnenolone leaves the mitochondria to become the obligatory precursor of corticosteroids and adrenal androgens. The biosynthetic pathway next branches into two separate routes. One route passes through proges-terone and corticosterone to aldosterone, and the other proceeds from 17 -hydroxyprogesterone and 1-deoxy-cortisol to yield cortisol.
Thus, steroid
intermediates are converted to steroid end products by sequential 17-, 21-, and
11-hydroxylation reactions. 11- -Hydroxylation is essential for glucocorticoid
and mineralocorticoid activ-ity of a steroid. The steroid hydroxylase system
has the characteristics of a mixed-function oxidase, since two substrates,
steroid and NADPH, are oxidized. All hy-droxylases seem to be associated with a
specific cy-tochrome P450.
The 17- and 21-hydroxylase
enzymes are associated with microsomes, whereas the 11- -hydroxylase has a
mitochondrial origin. Since the last-named enzyme is not detectable in other
steroid-producing tissues, the term 11-oxygenated steroids is considered
synonymous with adrenal steroids. Aldosterone synthesis involves an essential
18-hydroxylation step catalyzed by P450c18 with corticosterone as
the precursor; this reaction also takes place within the mitochondria.
Steroid Transport in Blood
Glucocorticoids secreted into
the systemic circulation are reversibly bound to a specific -globulin known as transcortin or corticosteroid-binding globulin. This bind-ing system has a high
affinity and low capacity for corti-costeroids, which contrasts with the
low-affinity binding of these compounds to plasma albumin. Approximately 80% of
the normal cortisol content in human plasma (12 g/dL) is bound to
corticosteroid-binding globulin, while 10% is bound to serum albumin; the
remaining 10% is the biologically active unbound hormone.
Transcortin acts as a
reservoir from which a constant supply of unbound cortisol may be provided to
target cells. In addition, when serum albumin levels are low, less circulating
cortisol becomes bound, which yields a greater physiological effect. Not only
does protein bind-ing control the amount of biologically active cortisol
available, but it also reduces the rate at which steroids are cleared from the
blood and thus limits steroid sup-pression of corticotrophin release from the
pituitary gland.
The binding affinity of human
transcortin is not limited to corticoids. Progesterone and the synthetic
glucocorticoid prednisone also can bind to this macro-molecule. High estrogen
states (pregnancy, estrogen ad-ministration, use of oral contraceptives)
greatly in-crease circulating transcortin levels. Thyroxine also stimulates
transcortin formation, while androgen ad-ministration will decrease transcortin
levels and the amount of bound glucocorticoids.
Steroid Metabolism
Most of the cortisol
circulating in the blood is metabo-lized before its excretion. The metabolism
of adrenal steroids occurs primarily in the liver, and when meta-bolic
processes are altered, as occurs in liver disease, the half-life of cortisol
may increase from 100 minutes to 7 hours.
Two major steps are involved
in the metabolism of cortisol. The first is reduction of double bonds and
in-troduction of a hydroxyl group in the A ring to form tetrahydric
derivatives; this pathway accounts for 20 to 30% of the cortisol excreted. The
glucocorticoid-metabolizing microsomal enzymes 11 -hydroxysteroid
dehydrogenases (11 β-HSD) play a crucial role in deter-mining the availability
of glucocorticoids. 11 β-HSD-1 acts as a reductase, regenerating active
glucocorticoids, whereas 11 β-HSD-2 acts as a dehydrogenase, convert-ing
cortisol to its inactive 11-keto derivative (cortisone). By inactivating
glucocorticoids, 11 β-HSD-2 protects the mineralocorticoid receptor from
occupation by gluco-corticoids, thereby endowing specificity to the
aldo-sterone regulatory effects despite the predominance of glucocorticoids in
the circulation. By contrast, congeni-tal deficiency of 11 β-HSD-2 results in
inappropriate ac-tivation of the mineralocorticoid receptor by cortisol,
leading to hypertension and hypokalemia. The second step in the metabolism of
cortisol is a glucuronic acid or sulfate conjugation to form more soluble
derivatives that are poorly bound to plasma proteins and readily pass into the
urine. Adrenal androgens also are ex-creted, primarily as sulfates; they
constitute about two-thirds of the total urinary 17-ketosteroids excreted. In
the male, the other third is contributed by gonadal se-cretions. Knowledge of
corticosteroid metabolism is im-portant to the clinician, since alterations in
adrenocorti-cal function can be determined by measuring the amounts of
17-hydroxycorticosteroids. However, ra-dioimmunoassay of urinary free cortisol
(and plasma cortisol) is supplanting measurements of urinary metabolites.
Since the metabolism of
steroid hormones occurs in part through the action of the hepatic oxidative
drug-metabolizing enzymes, concomitant administration of anticonvulsant drugs
(e.g., phenytoin and carbamaze-pine), which are potent inducers of
glucocorticoid me-tabolism, will augment the elimination of methylpred-nisolone
severalfold. Also, since steroids such as prednisone lack glucocorticoid
activity until converted to prednisolone by hepatic enzymes, patients with
liver disease should be treated with prednisolone rather than prednisone.
Related Topics