Chapter: Modern Pharmacology with Clinical Applications: Adrenocortical Hormones and Drugs Affecting the Adrenal Cortex
Actions of the Corticosteroids
ACTIONS OF THE
CORTICOSTEROIDS
The pharmacological actions
of steroids are generally an extension of their physiological effects. Adrenal
cor-ticosteroids exert effects on almost every organ in the body. In normal
physiological concentrations, they are essential for homeostasis, for coping
with stress, and for the very maintenance of life.
The designation
“glucocorticoid activity” is arbi-trary, since naturally occurring
glucocorticoids, such as cortisol, also possess mineralocorticoid activity, and
the principal mineralocorticoid, aldosterone, when adminis-tered in very high
doses, has glucocorticoid activity. Moreover, hydrocortisone, as well as
certain synthetic glucocorticoids, such as prednisone and dexametha-sone, binds
to mineralocorticoid receptors. However, the distinction between these two
groups serves a use-ful purpose when dissociation of the basic actions be-comes
crucial for optimizing steroids’ therapeutic effi-ciency.
Carbohydrate, Protein, and Fat Metabolism
The glucocorticoids increase
blood glucose and liver glycogen levels by stimulating gluconeogenesis. The
source of this augmented carbohydrate production is protein, and the protein
catabolic actions of the gluco-corticoids result in a negative nitrogen balance.
The in-hibition of protein synthesis by glucocorticoids brings about a transfer
of amino acids from muscle and bone to liver, where amino acids are converted
to glucose.
Supraphysiological
concentrations of glucocorti-coids will induce the synthesis of specific
proteins in various tissues. For instance, glucocorticoids stimulate the
synthesis of enzymes involved in glucose and amino acid metabolism, including
glucose 6-phosphatase and tyrosine transaminase. The relation of this action of
glu-cocorticoids to their overall effects on general meta-bolic processes
remains obscure, although the latency of their therapeutic actions (several
hours) is consistent with the fact that steroids regulate RNA and protein
synthesis.
Glucocorticoids not only
break down protein but also stimulate the catabolism of lipids in adipose
tissue and enhance the actions of other lipolytic agents. This occurrence
results in an increase in plasma free fatty acids and an enhanced tendency to
ketosis. The mecha-nism of this lipolytic action is unknown. The net effect of
the biochemical changes induced by the glucocorti-coids is antagonism of the
actions of insulin. These bio-chemical events promote hyperglycemia and
glycosuria, which are similar to the diabetic state.
Electrolyte and Water Metabolism
Another major function of the
adrenal cortex is the reg-ulation of water and electrolyte metabolism. The
princi-pal mineralocorticoid, aldosterone, can increase the rate of sodium
reabsorption and potassium excretion sever-alfold. This will occur
physiologically in response to sodium or volume depletion or both. The primary
site of this effect is the distal tubule . The steroid-binding specificity of
mineralocorticoid and glu-cocorticoid receptors overlaps in the distal cortical
cells and collecting tubules, so that glucocorticoids may medi-ate
mineralocorticoid-like effects. Glucocorticoids also decrease the intestinal
transport of calcium by antago-nizing the action of 1,25-dihydroxyvitamin D3
and pro-mote calcium excretion by the kidney .
Cardiovascular Function
Glucocorticoids directly
stimulate cardiac output and potentiate the responses of vascular smooth muscle
to the pressor effects of catecholamines and other vaso-constrictor agents.
Such actions on vascular smooth muscle may be secondary to effects mediated
through the central nervous system or on circulating volume. However, the
presence of steroid receptors on vascular smooth muscle suggests a direct
effect on vasomotor ac-tivity. Thus,
corticosteroids appear to play an important role in the regulation of blood pressure by modulating vascular smooth
muscle tone, by having a direct action on the heart, and through stimulating
renal mineralocor-ticoid and glucocorticoid receptors. The resulting
hyper-tension may predispose patients to coronary heart dis-ease if a prolonged
course of rigorous glucocorticoid therapy is employed.
Immune and Defense Mechanisms
The inflammatory response is
a highly complex process that involves a number of cell types of the
reticuloen-dothelial system and a number of chemical mediators, including
prostaglandins, leukotrienes, kinins, and bio-genic amines . The inhibitory
effects of glucocorticoids on various aspects of the inflammatory and
immunological responses constitute the basis for their therapeutic efficacy.
All steps of the inflammatory process are blocked: there is a diminution in
heat, ery-thema, swelling, and tenderness. Both the early compo-nents (edema,
fibrin deposition, neutrophil migration, and phagocytosis) and late components
(collagen syn-thesis and deposition) may be retarded.
Glucocorticoids promote
apoptosis and reduce survival, differentiation, and proliferation of a variety
of inflammatory cells, including T lymphocytes and macrophages. These effects
are mediated by changes in the production and activity of inflammatory
cytokines, such as interleukin (IL) 6 and IL-β , tumor necrosis fac-tor- , and interferon-γ . Many of the
antiinflammatory actions of glucocorticoids are mediated by cross-talk between
the activated glucocorticoid receptor and tran-scription factors, such as the
proinflammatory nuclear factor-κ -B (NF- κ B) and activator protein (AP) 1. These transcription factors,
which promote the expression of a number of inflammatory genes, are potential
targets for antiinflammatory therapy as observed in asthma, for example.
A prominent histological
feature of glucocorticoid action on the late-phase response to bronchial
inhala-tion challenge with antigen is inhibition of the influx of
polymorphonuclear leukocytes, eosinophils, basophils, mononuclear cells, and
lymphocytes into tissues (Fig. 60.2). The ability of glucocorticoids to alter
reticuloen-dothelial cell traffic, which is a prominent antiinflamma-tory
action of glucocorticoids, is regulated by adhesion molecules. Glucocorticoids
reduce the expression of ad-hesion molecules through the inhibition of
proinflammatory cytokines and by direct inhibitory effects on the expression of
adhesion molecules. Chemotactic cy-tokines, such as IL-8, which attract immune
cells to the inflammatory site, are also inhibited by glucocorticoids. In
addition to their ability to inhibit the adherence of inflammatory cells,
particularly neutrophils, to the vas-cular endothelium, steroids are
vasoconstrictors. This action would further impede inflammatory cell migra-tion
into tissues.
As mentioned previously,
glucocorticoids promote apoptosis and reduce survival, differentiation, and
pro-liferation of a number of inflammatory cells. While there is an increase in
the number of polymorphonu-clear leukocytes in the circulation, corticosteroids
cause the involution and atrophy of all lymphoid tissue and decrease the number
of circulating lymphocytes. The striking lymphocytopenia is caused in large
part by an inhibition of lymphocyte proliferation, although dimin-ished growth
with preferential accumulation of cells in the G1-phase of the cell
cycle is followed by cell death. These effects are mainly mediated by
alterations in cy-tokine production and action.
Another important aspect of
the inflammatory cas-cade is arachidonic acid metabolism, leading to the
synthesis of the proinflammatory prostaglandins and leukotrienes. Through the
formation of lipocortin, an in-hibitor of phospholipase A2,
glucocorticoids depress the release of arachidonic acid from phospholipids and
hence the production of arachidonic acid metabolites.
Other Endocrine Organs
Since the synthesis and
release of cortisol are regulated by pituitary corticotrophin, removal of the
pituitary gland results in decreased function and eventual atro-phy of the zona
fasciculata and zona reticularis. Infusion of supraphysiological concentrations
of cortisol will suppress corticotrophin secretion from the pituitary and will
markedly decrease circulating corticotrophin levels. This occurrence implies a
negative feedback control for corticotrophin and corticosteroid release (Fig.
60.3).
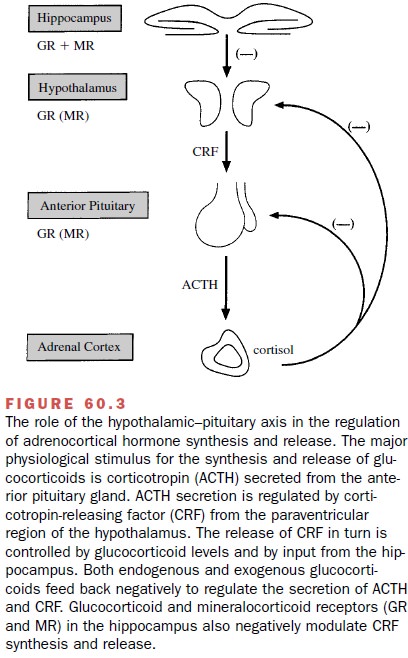
In addition to the humoral control of corticotrophin release, direct nervous control is mediated through the median eminence of the hypothalamus (Fig. 60.3). Nerve terminals in the median eminence store and re-lease various hormones and neurotransmitters, includ-ing corticotropin-releasing factor (CRF), which is under the control of higher neural centers. During stress, CRF is released into the pituitary portal system to stimulate corticotrophin release. Activation of the hypothala-mic–pituitary system also accounts for the diurnal, or circadian, nature of cortisol secretion; plasma cortisol concentrations reach a maximum between 6 and 8 A.M. and then slowly decrease through the afternoon and evening. Human and animal studies suggest the exis-tence of an early (fast) and more prolonged (delayed, > 2 hours) feedback of corticotrophin suppression.
Both inhibitory systems are operative
at the hypothalamic and pituitary levels. The hippocampus also highly
ex-presses glucocorticoid and mineralocorticoid receptors, which when
activated, decrease the synthesis and re-lease of CRF. This results in a
decrease in basal and corticotrophin-induced cortisol secretion (Fig. 60.3).
Corticosteroids also affect
adrenomedullary func-tion by increasing epinephrine production; the mecha-nism
is exertion of a stimulatory action on two of the enzymes that regulate
catecholamine synthesis, tyrosine hydroxylase, the rate-limiting enzyme, and
phenyl-ethanolamine N-methyltransferase,
which catalyzes the conversion of norepinephrine to epinephrine. Steroids also
influence the metabolism of circulating cate-cholamines by inhibiting their
uptake from the circula-tion by nonneuronal tissues. This effect of corticoids
may explain their permissive action in potentiating the hemody-namic effects of
circulating catecholamines.
Finally, steroids can exert
suppressive actions on cer-tain endocrine systems. Glucocorticoids inhibit
thyroid-stimulating hormone pulsatility and the nocturnal surge of this hormone
by depressing thyrotropin-releasing hormone secretion at the hypothalamic
level. In addi-tion to hypercortisolism being associated with insulin
resistance, glucocorticoids are inhibitors of linear growth and skeletal
maturation in humans. A pivotal component of this inhibition is the depression
of growth hormone secretion. The anticalcemic effect of the glu-cocorticoids,
which is associated with an amplification of the actions of parathyroid
hormone, also may retard bone growth. The inhibitory action of high levels of
glu-cocorticoids on reproductive function is probably be-cause of attenuation
of luteinizing hormone secretion and direct action on the reproductive organs.
Related Topics