Chapter: Modern Pharmacology with Clinical Applications: Adrenocortical Hormones and Drugs Affecting the Adrenal Cortex
Adverse Effects
ADVERSE EFFECTS
General Considerations
Short-term glucocorticoid
therapy of life-threatening diseases, such as status asthmaticus, provides
dramatic improvement with few complications. However, when administered in pharmacological
doses for long periods, steroids generally produce serious toxic effects that
are extensions of their pharmacological actions. No route or preparation is
free from the diverse side effects (Table 60.2), although individuals receiving
comparable doses of glucocorticoids exhibit variations in side effects.
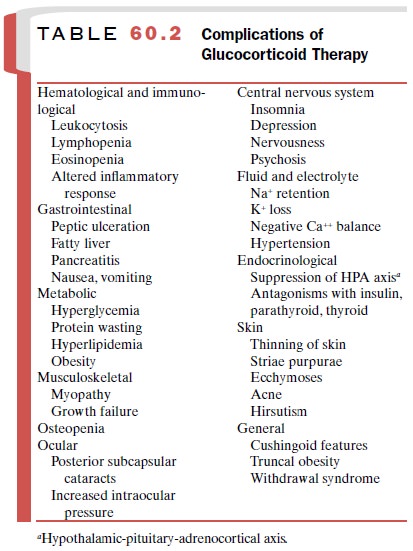
Glucocorticoids are
cautiously employed in various disease states, such as rheumatoid arthritis,
although they still should be regarded as adjunctive rather than primary
treatment in the overall management scheme. The toxic effects of steroids are
severe enough that a number of factors must be considered when their pro-longed
use is contemplated.
The first point is that
treatment with steroids is gen-erally palliative rather than curative, and only
in a very few diseases, such as leukemia and nephrotic syndrome, do
corticosteroids alter prognosis. One must also con-sider which is worse, the
disease to be treated or possi-ble induced hypercortisolism. The patient’s age
can be an important factor, since such adverse effects as hyper-tension are
more apt to occur in old and infirm individ-uals, especially in those with
underlying cardiovascular disease. Glucocorticoids should be used with caution
during pregnancy. If steroids are to be employed, pred-nisone or prednisolone
should be used, since they cross the placenta poorly.
Once steroid therapy is decided upon, the lowest possible dose that can provide the desired therapeutic effect should be employed. Relationships of dosage, du-ration, and host responses are essential elements in de-termining adverse effects. Increasing attention is being given to the use of lower doses of glucocorticoids in combination with other drugs that can have a synergis-tic effect on a given disease. Moreover, the lowered dose levels of steroid will minimize the side effects.
Osteoporosis
The most damaging and
therapeutically limiting ad-verse effect of long-term glucocorticoid therapy is
im-pairment of bone formation. This effect is associated with a decrease in
serum levels of osteocalcin, a marker of osteoblastic function. In fact, glucocorticoid adminis-tration is the most
common cause of drug-induced osteo-porosis. Most patients receiving chronic
steroid therapy develop osteoporosis,
particularly during the first year of therapy, and more than 50% will have a
bone frac-ture. Trabecular bone is particularly affected.
Systemic glucocorticoid
therapy increases the prob-ability of osteoporosis even with dosages
sufficiently low so as not to affect the hypothalamic–pituitary– adrenal axis.
By enhancing bone resorption and de-creasing bone formation, glucocorticoids
decrease bone mass and increase the risk of fractures. The overall ef-fects
appear to be due to direct actions of glucocorti-coids on osteoblasts and to
indirect effects, such as im-paired Ca++ absorption and a
compensatory increase in parathyroid hormone secretion. Inhibition of bone
growth is a well-known side effect of long-term systemic glucocorticoid therapy
in children with bronchial asthma, even in those receiving alternate-day
therapy. Glucocorticoids can also augment bone loss, decreasing testosterone
levels in men and estrogen levels in women by direct effects on the gonads and
inhibition of go-nadotropin release. Thus, patients taking glucocorti-coids can
also develop hypogonadism. It is recom-mended that all patients who receive
long-term glucocorticoid treatment should have measurements of bone density,
gonadal steroids, vitamin D, and 24-hour urinary Ca++ . Deficiencies
in either testosterone or estradiol increase bone loss and should be corrected
if possible. Bisphosphonates (etidronate, alendronate, or risedronate) and
calcitonin, which inhibit bone resorp-tion, have become increasingly popular
for treating os-teoporosis.
The Infectious Process
Steroids can alter
host–parasite interactions, suppress fever, decrease inflammation, and change
the usual character of the symptoms produced by most infectious organisms.
There is a heightened susceptibility to seri-ous bacterial, viral, and fungal
infections. Local infec-tions may reactivate and spread, and infections
ac-quired during the course of therapy may become more severe and even more
difficult to recognize. By interfer-ing with fibroblast proliferation and
collagen synthesis, glucocorticoids cause dehiscence of surgical incisions,
increase risk of wound infection, and delay healing of open wounds. This
untoward effect of steroids may make it mandatory to administer antibiotics
with the steroids, especially when there is a history of a chronic infectious
process (e.g., tuberculosis). On the other hand, individuals with normal
defenses who are treated with low to moderate doses of glucocorticoids are not
at great risk of infection. While the incidence of infections has probably decreased
with the increased use of in-haled steroids and combination therapy, inhaled
steroids carry an increase in the incidence of oral can-didiasis that can be
reduced by using proper doses. Nevertheless, glucocorticoids are used to treat
herpes zoster, bacterial meningitis, and skin infections.
Effects on Gastric Mucosa
Steroid administration was
once thought to lead to the formation of peptic ulcers, with hemorrhage or
perfora-tion or reactivation of a healed ulcer. It is now realized that this
effect is principally observed in patients who have received concomitant
nonsteroidal antiinflamma-tory treatment. Since there is a minimal increase in
the incidence of ulcers in patients receiving glucocorticoid treatment alone,
prophylactic antiulcer regimens are usually not necessary.
Hyperglycemic Action
In about one-fourth to
one-third of the patients receiv-ing prolonged steroid therapy, the
hyperglycemic effects of glucocorticoids lead to decreased glucose tolerance,
decreased responsiveness to insulin, and even glyco-suria. Ketoacidosis occurs
very rarely. Pharmacological concentrations of steroids may precipitate frank
dia-betes in individuals who cannot produce the necessary additional insulin.
Mild hyperglycemia can often be managed with oral hypoglycemic agents. The
effects of glucocorticoids on hyperglycemia are usually reversed within 48
hours following discontinuation of steroid therapy. If glucocorticoid therapy
is continued for an extended period, the alterations of glucose metabolism and
the resulting hyperinsulinemia may lead to en-hanced cardiovascular risk.
Ophthalmic Effects
Glucocorticoids induce
cataract formation, particularly in patients with rheumatoid arthritis. An
increase in intraoc-ular pressure related to a decreased outflow of aqueous
humor is also a frequent side effect of periocular, topical, or systemic
administration. Induction of ocular hyperten-sion, which occurs in about 35% of
the general population after glucocorticoid administration, depends on the
spe-cific drug, the dose, the frequency of administration, and the
glucocorticoid responsiveness of the patient.
Central Nervous System Effects
Treatment with steroids may
initially evoke euphoria. This reaction can be a consequence of the salutary
ef-fects of the steroids on the inflammatory process or a di-rect effect on the
psyche. The expression of the unpre-dictable and often profound effects exerted
by steroids on mental processes generally reflects the personality of the
individual. Psychiatric side effects induced by gluco-corticoids may include
mania, depression, or mood dis-turbances. Restlessness and early-morning
insomnia may be forerunners of severe psychotic reactions. In such situations,
cessation of treatment might be consid-ered, especially in patients with a history
of personality disorders. In addition, patients may become psychically
dependent on steroids as a result of their euphoric ef-fect, and withdrawal of
the treatment may precipitate an emotional crisis, with suicide or psychosis as
a conse-quence. Patients with Cushing’s syndrome may also ex-hibit mood
changes, which are reversed by effective treatment of the hypercortisolism.
The hippocampus is a
principal neural target for glu-cocorticoids. It contains high concentrations
of gluco-corticoid and mineralocorticoid receptors and has marked sensitivity
to these hormones.
Fluid and Electrolyte Disturbances
The normal subject may retain sodium and water
during steroid therapy, although the synthetic steroid analogues represent a
lesser risk in this regard. Pred-nisolone produces some edema in doses greater
than 30 mg; triamcinolone and dexamethasone are much less li-able to elicit
this effect. Glucocorticoids may also pro-duce an increase in potassium
excretion. Muscle weak-ness and wasting of skeletal muscle mass frequently
accompany this potassium-depleting action. The expan-sion of the extracellular
fluid volume produced by steroids is secondary to sodium and water retention.
However, the presence of specific steroid receptors in vascular smooth muscle suggests
that glucocorticoids are also more directly involved in the regulation of blood
pressure. The major adverse effects of glucocorti-coids on the cardiovascular
system include dyslipidemia and hypertension, which may predispose patients to
coronary artery disease. A separate entity, steroid my-opathy, is also improved
by decreasing steroid dosage.
Pseudorheumatism
In certain patients, whose
large dosages of cortico-steroids for rheumatoid arthritis are gradually
dimin-ished, new symptoms develop that may be mistaken for a flare-up of the
joint disease. These can include emo-tional lability, fever, muscle aches, and
general fatigue. It is tempting to increase the dosage of steroid in this
sit-uation, but continued maintenance at the lower dosage with a subsequent
gradual decrease in the dose usually improves symptoms.
Additional Effects
Other side effects include
acne, striae, truncal obesity, deposition of fat in the cheeks (moon face) and
upper part of the back (buffalo hump), and dysmenorrhea. Topical administration
may produce local skin atrophy. In patients with AIDS who are treated with
glucocorti-coids, Kaposi’s sarcoma becomes activated or pro-gresses more
rapidly.
Iatrogenic Adrenal Insufficiency
In addition to the dangers
associated with long-term use of corticosteroids in supraphysiological
concentrations, withdrawal of steroid therapy presents problems. The
suppression of the hypothalamic–pituitary axis ob-served with modest doses and
short courses of gluco-corticoid therapy is usually readily reversible.
However, steroid therapy with modest to high doses for 2 weeks or longer will
depress hypothalamic and pituitary activ-ity and result in a decrease in
endogenous adrenal steroid secretion and eventual adrenal atrophy. These
patients have a limited ability to respond to stress and an enhanced
probability that shock will develop. Long-acting steroids, such as
dexamethasone and betametha-sone, suppress the hypothalamic–pituitary axis more
than do other steroids. The
functional state of the hypo-thalamic–pituitary axis can be evaluated by tests
involv-ing basal plasma cortisol determinations, low and high doses of
cosyntropin (peptide fragment of corti-cotrophin), insulin hypoglycemia,
metyrapone, and corticotrophin-releasing hormone.
Glucocorticoids are not
withdrawn abruptly but are tapered. The doses are altered so that the condition
being treated will not flare up and recovery of the hypothalamic–pituitary axis
will be facilitated. Tapering the dose may reduce the potential for the development
of Addison-like symptoms associated with steroid with-drawal. Alternate-day
therapy will relieve the clinical manifestations of the inflammatory diseases
while allow-ing a day for reactivation of endogenous corticosteroid output,
thereby causing less severe and less sustained hypothalamic–pituitary
suppression. This is feasible with doses of shorter-acting corticosteroids,
such as pred-nisolone. The usual daily dose is doubled and is given in the
early morning to simulate the natural circadian vari-ation that occurs in
endogenous corticosteroid secretion. The benefits of alternate-day therapy are
seen only when steroids are used for a long period and are partic-ularly useful
for tapering the dose of glucocorticoid.
Although not always
predictable, the degree to which a given corticosteroid will suppress pituitary
activity is re-lated to the route of administration, the size of the dose, and
the length of treatment. The parenteral route causes the greatest suppression,
followed by the oral route, and finally topical application.
Hypothalamic–pituitary sup-pression also may result if large doses of a steroid
aero-sol spray are used to treat bronchial asthma. Patients given high
concentrations of steroids for long periods and subsequently exposed to undue stress
(e.g., severe infection, surgery) face the danger of adrenal crisis. These
patients must be given supplemental steroids to compensate for their lack of
adrenal reserve and to sus-tain them during the crisis.
Acute adrenal insufficiency
will, of course, occur from an abrupt cessation of steroid therapy. The
causa-tion of fever, myalgia, arthralgia, and malaise may be difficult to
distinguish from reactivation of rheumatic disease. Steroid treatment should be
reduced gradually over several months to avoid this potentially serious
problem. Also, continued suppression may be avoided by administering daily
physiological replacement doses (5 mg prednisone) until adrenal function is
restored. Although tapering of dose may not facilitate recovery of the hypothalamic–pituitary–adrenal
axis, it may re-duce the possibility of adrenal insufficiency. This is
im-portant, since severe hypotension caused by adrenal in-sufficiency may evoke
a medical emergency. Adrenal insufficiency should always be considered in patients
who are being withdrawn from prolonged glucocorti-coid therapy unless
metyrapone or insulin hypo-glycemia tests are performed to exclude this
possibility.
An additional problem
associated with glucocorti-coid therapy is that certain side effects can be
caused by the diseases for which glucocorticoids are adminis-tered. Thus,
osteoporosis can be a sequela of rheuma-toid arthritis, and the physician is
left to determine whether the untoward effect is iatrogenic or is merely a sign
of the disease being treated. In addition to these problems, the physician must
also be aware of the pa-tient’s natural reluctance to reduce the dose of
steroid because of its salutary effects, both on the inflamma-tory process and
on the psyche. Thus, the problems as-sociated with withdrawal from long-term
steroid ther-apy in rheumatoid arthritis are additional reasons steroid
treatment should be initiated only after rest, physiotherapy, and nonsteroidal
antiinflammatory drugs or after methotrexate, gold, and D-penicillamine have been
used.
Related Topics