Chapter: Modern Pharmacology with Clinical Applications: Adrenocortical Hormones and Drugs Affecting the Adrenal Cortex
General Pharmacology of Corticosteroids
GENERAL PHARMACOLOGY
OF CORTICOSTEROIDS
Structure-Activity Relationships
Natural Corticosteroids
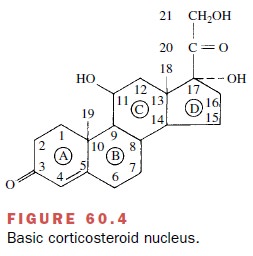
Within the basic structure of
the steroid molecule (Fig. 60.4), a 4,5 double bond and a 3-ketone group are
needed for typical steroid activity. A hydroxyl group on C11 is needed for
glucocorticoid activity (cortico-sterone) but is not required for
sodium-retaining activ-ity (desoxycorticosterone). The addition of a hydroxyl
group on C17, which converts corticosterone to cortisol, also increases
glucocorticoid activity.
Synthetic Corticosteroids
The ultimate aim in altering
the steroid molecule is to decrease sodium-retaining activity and to increase
anti-inflammatory glucocorticoid activity.
Ring A
The addition of a double bond at the 1,2 position of cortisol or cortisone yields prednisone or prednisolone, respectively, and increases the ratio of carbohydrate to sodium-retaining potency. Prednisone is inactive and must be converted to prednisolone in the liver by re-duction at the 11-keto position.
Ring B
The inclusion of an -methyl
group in position 6 of prednisolone will yield 6- -methylprednisolone, a
com-pound with slightly greater glucocorticoid potency. This small structural
modification greatly diminishes the binding of methylprednisolone to transcortin.
Ring C
The addition of a fluoride
group on the 9 position of cortisol to give 9- α -fluorocortisol will greatly increase all
biological activity.
Ring D
Hydroxylation or methylation
at the 16 position of α-fluoroprednisolone to give triamcinolone, dexam-ethasone, or
betamethasone increases antiinflammatory potency and drastically diminishes
sodium-retaining activity.
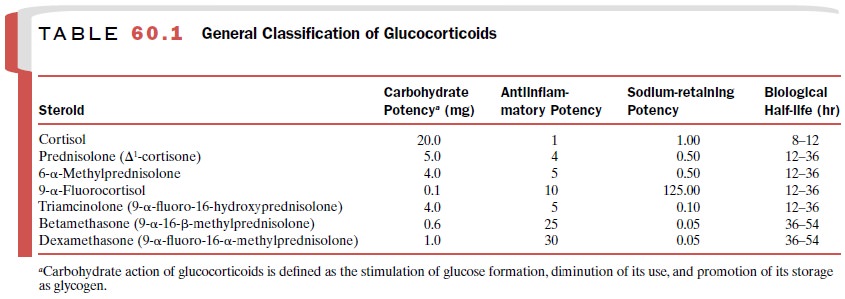
The relative antiinflammatory
potency of each of the synthetic analogues is compared with cortisol in Table
60.1 and is roughly correlated with its biological half-life. Hydrocortisone is
considered a short-acting steroid; triamcinolone and prednisolone,
intermediate-acting; and betamethasone and dexamethasone, long-acting. Thus,
prednisone 5 mg, dexamethasone 0.75 mg, and hydrocortisone 20 mg should possess
equal gluco-corticoid potency.
The synthetic analogues
(except 9- α -fluorocortisol) share an advantage over hydrocortisone in that
sodium retention is not as marked at equipotent antiinflamma-tory doses.
However, all of the other undesirable side effects of supraphysiological
concentrations of hydro-cortisone have been observed with the synthetic
ana-logues.
Steroid Preparations
Glucocorticoids are available
in a wide range of prepa-rations, so that they can be administered
parenterally, orally, topically, or by inhalation. Obviously the oral route is
preferred for prolonged therapy. However, par-enteral administration is
required in certain circum-stances. Intramuscular injection of a water-soluble
ester (phosphate or succinate) formed by esterification of the C21 steroid
alcohol produces peak plasma steroid levels within 1 hour. Such preparations
are useful in emergen-cies. By contrast, acetate and tertiary butylacetate
esters must be injected locally as suspensions and are slowly absorbed from the
injection site, which prolongs their effectiveness to approximately 8 hours.
Topical preparations usually
contain relatively insol-uble steroids, such as clobetasol propionate,
triamcin-olone acetonide, or triamcinolone diacetate. Side effects of this mode
of drug application are usually milder and more transient than those seen after
systemically ad-ministered steroids. However, potent topical cortico-steroids,
such as clobetasol propionate (Temovate),
can suppress adrenal function when used in large amounts for a long time,
especially when the skin surface is de-nuded or when occlusive dressings are
employed. Since the high potency topical preparations carry a higher risk of
local side effects, their use should be held in reserve.
Inhaled glucocorticoid preparations, such as be-clomethasone dipropionate and betamethasone valer-ate, provide an effective alternative to systemic steroids in the treatment of chronic asthma, with lesser side ef-fects than oral or parenteral glucocorticoids . In fact, inhaled glucocorticoids have be-come a mainstay of asthma therapy. Inhalation delivers the agent directly to the target site in relatively low doses, with the potential for more frequent administra-tion. Moreover, inhaled glucocorticoids are metabolized in the lung before they are absorbed, which reduces their systemic effects.
However, even modest doses of inhaled steroid can have a
measurable effect on the hypothalamic–pituitary axis, although the clinical ef-fects
may be marginal. There is also a close association in adults between the heavy
use of inhaled glucocorti-coids and the risk of posterior subcapsular cataract.
Since the dose–response curve to inhaled glucocorti-coids is relatively flat,
the use of steroid-sparing agents (long-acting inhaled β-adrenergic agonists,
low-dose theophylline, or antileukotrienes) is recommended in-stead of
increasing the dose of glucocorticoid. This strategy will also limit the
severity of hypothalamic–pi-tuitary–adrenal depression and other side effects.
Related Topics