Chapter: Modern Pharmacology with Clinical Applications: Antiinflammatory and Antirheumatic Drugs
Specific Nonsteroidal Antiinflammatory Drugs
Specific Nonsteroidal
Antiinflammatory Drugs
The acidic NSAIDs include the
salicylates and an in-creasing number of other compounds. The latter agents, as
a group, share many common properties: they may have toxicities, are highly
protein bound and have the potential for interacting with other protein-bound
drugs. The choice of a particular agent often depends on the reaction of the
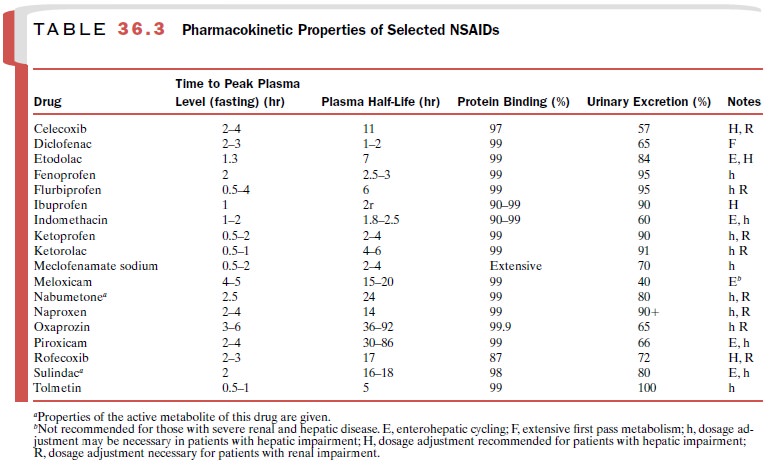
patient. Table 36.3 illustrates phar-macokinetic properties of selected NSAIDs.
Salicylates
Among the salicylates,
aspirin and sodium salicylate are by far the most commonly used.
The salicylates are useful in
the treatment of minor musculoskeletal disorders such as bursitis, synovitis,
tendinitis, myositis, and myalgia. They may also be used to relieve fever and
headache. They can be used in the treatment of inflammatory disease, such as
acute rheu-matic fever, rheumatoid arthritis, osteoarthritis, and cer-tain
rheumatoid variants, such as ankylosing spondylitis, Reiter’s syndrome, and
psoriatic arthritis. However, other NSAIDs are usually favored for the
treatment of these chronic conditions because of their lower inci-dence of GI
side effects. Aspirin is used in the treatment and prophylaxis of myocardial
infarction and ischemic stroke.
Basic Pharmacology
Aspirin is available as
capsules, tablets, enteric-coated tablets (Ecotrin),
timed-release tablets (ZORprin),
buffered tablets (Ascriptin, Bufferin),
and as rectal sup-positories. Sodium salicylate is available generically. Other
salicylates include choline salicylate (Arthropan),
choline magnesium trisalicylate (Trilisate),
and magne-sium salicylate (Momentum).
Although aspirin itself is
pharmacologically active, it is rapidly hydrolyzed to salicylic acid after its
absorp-tion, and it is the salicylate
anion that accounts for most of the
anti-inflammatory activity of the drug. The supe-rior analgesic activity of
aspirin compared with sodium salicylate implies that aspirin has an intrinsic
activity that is not totally explainable by its conversion to sali-cylic acid.
Aspirin inhibits COX-1 to a much greater ex-tent than COX-2; sodium salicylate
is more selective for COX-1. This, combined with the ability of aspirin to
acetylate proteins, might account for some of the thera-peutic and
toxicological differences between aspirin and the other salicylates.
The binding of salicylic acid
to plasma proteins varies with its plasma concentrations. At serum salicylic
acid concentrations of less than 100 μg/mL, 90 to 95% is protein bound; at 100
to 400 μg/mL, 70 to 85% is pro-tein bound; and at concentrations greater than
400 μg/mL, 20 to 60% is protein bound. The
plasma con-centration of salicylate that is associated with anti-inflammatory
activity (200–300 μg/mL) is about six times that needed to produce analgesia. At
these higher concentrations,
salicylate metabolism is reduced, result-ing in a longer half-life for the
drug. This reaction is a consequence of the saturable enzyme systems that
me-tabolize salicylates. The plasma half-life for salicylate has been estimated
to be 3 to 6 hours at the lower (anal-gesic) dosage and 15 to 30 hours at the higher
(anti-inflammatory) dosages. The rate of hydrolysis of aspirin to salicylic
acid is not dose limited, and no differences in the absorption of aspirin have
been observed between arthritic patients and normal individuals.
Adverse Effects
The most common adverse
effects produced by the salicylates are GI disturbances. Occult blood loss from
the GI tract, peptic ulceration, and rarely, severe GI hemorrhage can occur.
Because salicylic acid is highly bound to plasma proteins, it may be displaced
by other highly protein-bound drugs such as oral anticoagulants, sulfonylureas,
phenytoin, penicillins, and sulfonamides. The nonacetylated salicylates have
greatly reduced effects on blood loss and produce fewer adverse GI effects. In
addition, they may be somewhat kidney spar-ing. Salicylates may provoke
hypersensitivity reactions and prolonged bleeding time in some individuals.
Tinnitus, hearing impairment, blurred vision, and light-headedness are
indicators of toxic dosages. The use of
aspirin in conjunction with any other
NSAID is not rec-ommended because of the lack of evidence that such combinations increase efficacy and
because of the in-creased potential for an adverse reaction. Salicylates are
contraindicated in children with febrile viral illnesses because of a possible
increased risk of Reye’s syn-drome.
Aryl and Heteroarylakanoic Acid–Type Drugs
The prototypes of this large
class of NSAIDs are in-domethacin and ibuprofen. These drugs are indicated for
the relief of acute and chronic rheumatoid arthritis and osteoarthritis. In
addition, a number of drugs of this class are also useful in ankylosing
spondylitis, acute gouty arthritis, bursitis, and tendinitis.
Adverse reactions are common
with the use of these drugs but usually do not result in serious morbidity. GI
and CNS effects and prolonged bleeding may occur. Fluid retention, skin rashes,
and ocular toxicity also oc-cur, but with much lower frequency than with the
sali-cylates. The selectivity for COX-1 and COX-2 varies from drug to drug and
accounts for some of the differ-ences in toxicity. None of the agents seems to be clearly more efficacious than the others; however, they generally cause less GI
blood loss and fewer other adverse reac-tions than does aspirin, and the
overall incidence of ad-verse reactions may be lower with these drugs.
Indomethacin
Indomethacin (Indocin) is used in the treatment of
acute gouty arthritis, rheumatoid arthritis, ankylosing spondylitis, and
osteoarthritis. It is not recommended for use as a simple analgesic or
antipyretic because of its potential for toxicity. While indomethacin inhibits
both COX-1 and COX-2, it is moderately selective for COX- It produces more CNS
side effects than most of the other NSAIDs. Severe headache occurs in 25 to 50%
of patients; vertigo, confusion, and psychological distur-bances occur with
some regularity. GI symptoms also are more frequent and severe than with most
other NSAIDs. Hematopoietic side effects (e.g., leukopenia, hemolytic anemia,
aplastic anemia, purpura, thrombo-cytopenia, and agranulocytosis) also may
occur. Ocular effects (blurred vision, corneal deposits) have been ob-served in
patients receiving indomethacin, and regular ophthalmological examinations are
necessary when the drug is used for long periods. Hepatitis, jaundice, pan-creatitis,
and hypersensitivity reactions also have been noted.
Sulindac
Sulindac (Clinoril) is chemically related to
in-domethacin and is generally used for the same indica-tions. It is a prodrug
that is metabolized to an active sul-fide metabolite and an inactive
metabolite. The most frequently reported side effects are GI pain, nausea,
di-arrhea, and constipation. The incidence of these effects is lower than for
indomethacin, presumably because sulindac is a prodrug and thus the active
metabolite is not highly concentrated at the gastric mucosa. As with
indomethacin, a rather high incidence of CNS side ef-fects (dizziness,
headache) also occurs.
Tolmetin
Tolmetin (Tolectin) is indicated for the relief of
os-teoarthritis, rheumatoid arthritis, ankylosing spondylitis, and moderate
pain. It is ineffective in gouty arthritis for unknown reasons. Tolmetin can
inhibit both COX-1 and COX-2 but has a moderate selectivity for COX-1. The most
frequently reported side effects are GI distur-bance and CNS reactions (e.g.,
headache, asthenia, and dizziness). These effects are less frequently observed
than after aspirin or indomethacin use. Blood pressure elevation, edema, and
weight gain or loss have been associated with tolmetin administration. Tolmetin
me-tabolites in urine have been found to produce pseudo-proteinuria in some
laboratory tests.
Ketorolac
Ketorolac (Toradol), an NSAID chemically related to
indomethacin and tolmetin, is mainly used as an anal-gesic, not for the
treatment of inflammatory disease. It is available in oral, parenteral, and
topical formulations.
Etodolac
Etodolac (Lodine) is indicated for the treatment
of osteoarthritis, rheumatoid arthritis, and acute pain. It in-hibits COX-2
with slightly more selectivity than COX-1 and therefore produces less GI
toxicity than many other NSAIDs. Common adverse effects include skin rashes and
CNS effects.
Diclofenac
Diclofenac (Voltaren, Cataflam) is approved for use
in rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, dysmenorrhea,
and topically for the treat- ment of ocular inflammation and actinic keratosis.
Diclofenac exhibits approximately equal selectivity for COX-1 and COX-2. The
most common adverse reac-tions are GI disturbances and headache. A reversible
el-evation of serum transaminases occurs in 15% of pa-tients.
Ibuprofen
Ibuprofen (Advil, Motrin) is used as an analgesic
and antipyretic as well as a treatment for rheumatoid arthritis and
degenerative joint disease. The most fre-quently observed side effects are
nausea, heartburn, epigastric pain, rash, and dizziness. Incidence of GI side
effects is lower than with indomethacin. Visual changes and cross-sensitivity
to aspirin have been reported. Ibuprofen inhibits COX-1 and COX-2 about
equally. It decreases platelet aggregation, but the duration is shorter and the
effect quantitatively lower than with as-pirin. Ibuprofen prolongs bleeding
times toward high normal value and should be used with caution in pa-tients who
have coagulation deficits or are receiving an-ticoagulant therapy.
Fenoprofen
Fenoprofen (Nalfon) is chemically and
pharmaco-logically similar to ibuprofen and is used in the treat-ment of
rheumatoid arthritis, osteoarthritis, and mild to moderate pain. GI effects
such as dyspepsia and pain are most common, although dizziness, pruritus, and
pal-pitations may occur. GI bleeding, sometimes severe, has been reported, and
interstitial nephritis has been rarely associated with this drug. Concomitant
administration of aspirin decreases the biological half-life of fenopro-fen by
increasing the metabolic clearance of hydroxy-lated fenoprofen. Chronic
administration of pheno-barbital also decreases the drug’s half-life.
Naproxen
Naproxen (Naprosyn) also has pharmacological
properties and clinical uses similar to those of ibupro-fen. It exhibits
approximately equal selectivity for COX-1 and COX-2 and is better tolerated
than certain NSAIDs, such as indomethacin. Adverse reactions re-lated to the GI
tract occur in about 14% of all patients, and severe GI bleeding has been
reported. CNS com-plaints (headache, dizziness, drowsiness), dermatologi-cal
effects (pruritus, skin eruptions, echinoses), tinnitus, edema, and dyspnea
also occur.
Ketoprofen
Ketoprofen (Orudis) is indicated for use in
rheuma-toid and osteoarthritis, for mild to moderate pain, and in dysmenorrhea.
The most frequently reported side ef-fects are GI (dyspepsia, nausea, abdominal
pain, diar-rhea, constipation, and flatulence) and CNS related (headache,
excitation). Edema and increased blood urea nitrogen have also been noted in
more than 3% of patients. Ketoprofen can cause fluid retention and in-creases
in plasma creatinine, particularly in the elderly and in patients taking
diuretics.
Flurbiprofen
Flurbiprofen (Ansaid) is indicated for the treatment
of rheumatoid arthritis and osteoarthritis. Its half-life, longer than that of
many of the NSAIDs, allows for twice daily dosing. The most common adverse
effects of flurbiprofen are similar to those of the other acidic NSAIDs.
Flurbiprofen inhibits both COX isoforms about equally.
Oxaprozin
Oxaprozin (Daypro) is approved for the treatment of
osteoarthritis and rheumatoid arthritis. Its long half-life allows for once
daily dosing. The most frequently re-ported adverse effects of this drug are
nausea, vomiting, and dyspepsia.
Nabumetone
Nabumetone (Relafen) is approved for rheumatoid
arthritis, osteoarthritis, and pain management. Its long half-life allows for
once-daily dosing. Although this drug is a weak inhibitor of COX, it is
metabolized in the liver to 6-methoxy-2-naphthylacetic acid (6-MNA), a strong
COX inhibitor that is chemically similar to naproxen. As with most NSAIDs, GI
side effects are most commonly reported. The incidence of gastric ul-ceration
is lower with nabumetone than with many other NSAIDs. This is due to its nature
as a prodrug, not to COX-2 selectivity. Lower-bowel complaints, rashes, and CNS
disturbances are common adverse effects.
Sulfonylphenyl Derivatives
Celecoxib (Celebrex) and rofecoxib (Vioxx) are highly selective COX-2
inhibitors. Because of this, they produce
less erosion of the GI mucosa and cause
less inhibition of platelet aggregation than do the nonselective COX
in-hibitors. Short-term (6 months-to a year) clinical trials have shown that celecoxib and rofecoxib
produce less GI toxicity than nonselective NSAIDs. However, seri-ous GI
bleeding and ulceration have occurred in pa-tients taking these drugs, and
long-term prospective studies of their safety have yet to be completed. Like the nonselective NSAIDs, the selective COX-2 inhibitors can produce renal
side effects such as hypertension and edema.
Celecoxib is indicated for
the treatment of os-teoarthritis and rheumatoid arthritis. Its use is
con-traindicated in individuals with hypersensitivity to sul-fonamides or other
NSAIDs. It should be used with caution in persons with hepatic disease.
Interactions oc-cur with other drugs that induce CYP2C9 (e.g. ri fampin) or
compete for metabolism by this enzyme (e.g. fluconazole, leflunomide). The most
common adverse reactions to celecoxib are mild to moderate GI effects such as
dyspepsia, diarrhea, and abdominal pain. Serious GI and renal effects have
occurred rarely.
Rofecoxib is approved for the
treatment of os-teoarthritis, dysmenorrhea, and acute pain. The most common
adverse reactions to rofecoxib are mild to moderate GI irritation (diarrhea,
nausea, vomiting, dys-pepsia, abdominal pain). Lower extremity edema and
hypertension occur relatively frequently (about 3.5%). It is not metabolized by
CYP2C9, so rofecoxib should not be subject to some of the interactions seen
with celecoxib. However, its metabolism is increased by the coadministration of
rifampin, which acts as a nonspe-cific inducer of hepatic metabolism.
Oxicam-Type Drugs
The oxicams are as effective
as indomethacin, and their long half-life allows for once-daily dosing.
Piroxicam (Feldene) is indicated for
the treatment of rheumatoid arthritis and osteoarthritis. Piroxicam is a
nonspecific COX inhibitor that has a much higher affinity for COX-1 than COX-2.
This may account for the large proportion (over 30%) of patients receiving
long-term therapy who have reported side effects. Adverse GI re-actions have
been the most frequently reported side ef-fect, but edema, dizziness, headache,
rash, and changes in hematological parameters have also occurred in 1 to 6% of
patients. Piroxicam can cause serious GI
bleeding, ulceration, and
perforation, particularly in the elderly, if the recommended dosage is exceeded or if aspirin is be-ing taken
concurrently.
Meloxicam (Mobic), recently introduced for the treatment
of osteoarthritis, is also used for rheumatoid arthritis and certain acute
conditions. Although meloxi-cam is sometimes reported to be a selective COX-2
in-hibitor, it is considerably less selective than celecoxib or rofecoxib. Its
adverse effects are similar to those of piroxicam and other NSAIDs; however,
the frequency of GI side effects is lower for meloxicam than for pirox-icam and
several other NSAIDs.
Fenamate-Type Drugs
Two compounds of the fenamate
class of antiinflamma-tory drugs are marketed in the United States. Mefenamic
acid (Ponstel) is indicated only for
analgesia and primary dysmenorrhea when therapy will not ex-ceed 1 week.
Meclofenamate sodium (Meclomen) is
prescribed for rheumatoid arthritis and osteoarthritis.
The fenamates show no clear superiority in
anti-inflammatory activity and may produce more adverse effects than other
NSAIDs. Diarrhea may be severe enough to necessitate discontinuation of drug
use. Other adverse GI reactions include nausea, vomiting, abdominal pain,
bleeding, and peptic ulceration. Decreases in the hematocrit or hemoglobin
values oc-cur in approximately one-sixth of patients taking meclofenamic acid,
but these do not usually require dis-continuation of therapy. Because of the
rare possibility of drug-induced hemolytic anemia, hematological analyses
should be performed on patients receiving long-term therapy if anemia is
suspected.
Phenylbutazone-Type Drugs
The phenylbutazone-type drugs
include phenylbuta-zone, oxyphenbutazone, antipyrine, dipyrone, and aminopyrine.
The use of these drugs has decreased be-cause of their propensity to cause
blood dyscrasias. Only antipyrine, used in as otic drops with benzocaine (Otocalm), is available in the United
States today; phenylbutazone is used in Canada, and dipyrone is used in some
European countries.
Acetaminophen
Acetaminophen (Tylenol) is an effective antipyretic and
analgesic that is well tolerated at therapeutic doses. It has only weak
antiinflammatory activity; thus, it is not useful in the treatment of
rheumatoid arthritis and other inflammatory conditions.
Related Topics