Chapter: 9th Science : Heat
Specific Heat capacity
Specific
Heat capacity
You might have felt that
the land is cool in the morning and hot during day time. But, water in a lake
will be almost at a particular temperature both in the morning as well as in
the afternoon. Both are subjected to same amount of heat energy from the Sun,
but they react differently. It is because both of them have different properties.
In general the amount of heat energy absorbed or lost by a body is determined
by three factors.
1.
Mass of the body
2.
Change in temperature of the body
3.
Nature of the material of the body
We can understand this
from the following observations.
Observation:1
Quantity of heat
required to raise the temperature of 1 litre of water will be more than the
heat required to raise the temperature of 500 ml of water. If Q is the quantity
of heat absorbed and m is the mass of the body, then Q α m
Observation: 2
Quantity of heat energy
(Q) required to raise the temperature of 250 ml of water to 100˚C is more than
the heat energy required to raise the temperature to 500C. Here, Q α ΔT, where ΔT
is the change in temperature of the body.
Hence, from the above
two observations, heat lost or gained by a substance when its temperature
changes by ΔT is:
Q α m T
Q = mC ΔT ……..(1.1)
From the above
equations, the absolute temperature and energy of a system are proportional to
each other. The proportionality constant is the specific heat capacity (c) of
the substance.
In order to understand
the specific heat capacity of the substance, think of heating 500 ml of water
and 500 ml of oil. Which will be heated first? Why? It is because heat gained
by a body depends upon the nature of the substance. The capacity of a substance
to gain heat energy is denoted by the term specific heat capacity.
Mathematically it is derived from the equation (1.1) as, C Q/m ΔT
Thus, specific heat
capacity of a substance is defined as the amount of heat required to raise the
temperature of 1 kg of the substance by 1˚C or 1 K. The SI unit of specific
heat capacity is Jkg-1
K-1. The most commonly used units of specific heat capacity are J/kg˚C
and J/g0C.
Among all the
substances, water has the highest specific heat capacity and its value is 4200
J/kg˚C. So, water absorbs a large amount of heat for unit rise in temperature.
Thus, water is used as a coolant in car radiators and factories to keep engines
and other machinery parts cool. It is because of the same reason the
temperature of water in the lake does not change much during day time. Specific
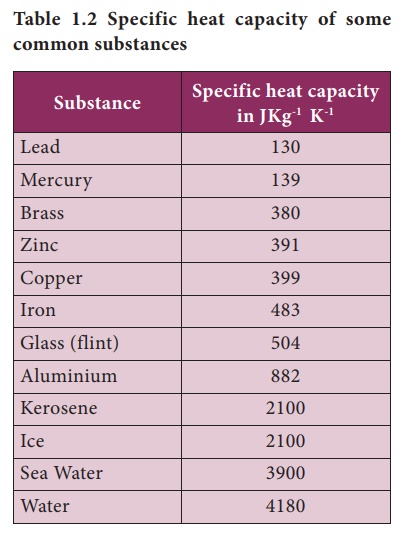
heat capacities of some common substances are given in Table 1.2.
Exercise 1.3
Calculate the heat
energy required to raise the temperature of 2kg of water from 10ºC to 50ºC.
Specific heat capacity of water is 4200 JKg-1 K-1.
Solution:
Given m 2 Kg, ΔT (50-10)
40ºC
Or in terms of Kelvin
(323.15-283.15) 40K, C 4200 J Kg-1 K-1
Heat energy required, Q
m x C x ΔT = 2 x 4200 x 40 = 3,36,000 J
Exercise 1.4
Some heat energy is
given to 120g of water and its temperature rises by 10K. When the same amount
of heat energy is given to 60g of oil, its temperature rises by 40K. The
specific heat capacity of water is 4200JKg-1 K-1.
Calculate:
i) The amount of heat
energy in joule given to water.
ii) The specific heat
capacity of oil.
Solution:
i. Heat energy given to
water = Mass of water x Specific heat capacity of water x rise in temperature.
= 120/1000 kg x 4200 JKg-1
K-1 10 K
= 5040 J.
ii. Since same heat
energy is given to oil, heat energy given to oil 5040 J
Let C in JKg-1
K-1 be the specific heat capacity of oil,
Then C = amount of heat
energy given to oil / [ mass of oil x rise in temperature]
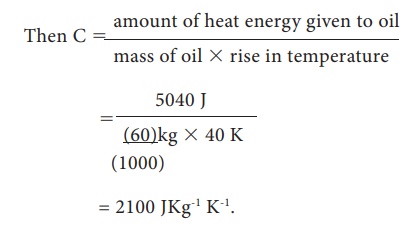
= 2100 JKg-1
K-1.
Related Topics