Chapter: 9th Science : Heat
Latent Heat
Latent
Heat
The word, ‘latent’ means
hidden. So, latent heat means hidden heat or hidden energy. In order to
understand latent heat, let us do the activity given below.
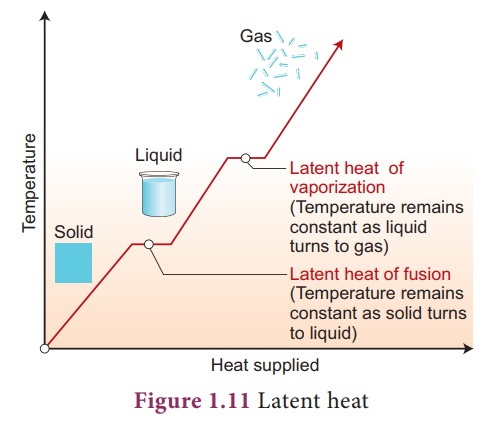
In the above activity,
the temperature is constant at 0˚C until entire ice is converted into liquid
and again constant at 100˚C until all the ice is converted into vapor. Why? It
is because, when a substance changes from one state to another, a considerable
amount of heat energy is absorbed or liberated. is energy is called latent
heat. us, latent heat is the
amount of
heat energy absorbed
or released by a substance during a change in its physical sates without any
change in its temperature.
Heat energy is absorbed
by a solid during melting and an equal amount of heat energy is liberated by
the liquid during freezing, without any temperature change. It is called latent
heat of fusion. In the same manner, heat energy is absorbed by a liquid during
vaporization and an equal amount of heat energy is liberated by the vapor
during condensation, without any temperature changes. is is called latent heat
of vaporization.
Specific latent heat
Latent heat when expressed
per unit mass of a substance, it is called specific latent heat. It is denoted
by the symbol L. If Q is the amount of heat energy absorbed or liberated by m
mass of a substance during its change of phase at a constant temperature, then
specific latent heat is given as L = Q/m.
Thus, specific latent
heat is the amount of heat energy absorbed or liberated by unit mass of a
substance during change of state without causing any change in temperature. The SI unit of specific
latent heat is J/kg.
Exercise 1.6
How much heat energy
is required to melt 5 kg of ice? (Speci c latent heat of ice = 336 Jg-1)
Solution:
Given, m = 5 Kg = 5000g, L 336 Jg-1
Heat energy required m x L
= 5000 x 336
= 1680000J or 1.68 x 106
J
Exercise 1.6
How much boiling water
at 100ºC is needed to melt 2kg of ice so that the mixture which is all water is
at 0ºC?
[Speci c heat capacity
of water 4.2 JKg-1 and speci c latent heat of ice 336 Jg-1].
Solution:
Given, Mass of ice = 2 kg = 2000 g.
Let m be the mass of boiling water
required.
Heat lost = Heat gained.
m x c x Δt = m x L
m x 4.2 x (100-0) = 2000 x 336
m = 2000x336 / 4.2x100
= 1600g or 1.6 kg.
Related Topics