Nuclear Physics | Science - Solved problem | 10th Science : Chapter 6 : Nuclear Physics
Chapter: 10th Science : Chapter 6 : Nuclear Physics
Solved problem
Nuclear Physics (Science)
Solved problem 6.1
Identify A, B, C, and D from the following nuclear reactions.
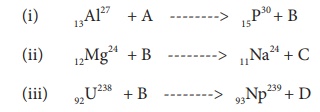
Solution:
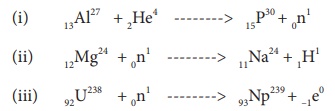
A is alpha particle, B is neutron, C is proton, and D is electron.
Solved problem 6.2
A radon specimen emits radiation of 3.7 × 103 GBq per second. Convert this disintegration in terms of curie. (one curie = 3.7 × 1010 disintegration per second)
1 Bq = one disintegration per second one curie = 3.7 × 1010 Bq
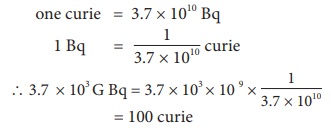
Solved problem 6.3
92 U 235 experiences one α - decay and one β - decay. Find number of neutrons in the final daughter nucleus that is formed.
Solution:
Let X and Y be the resulting nucleus after the emission of the alpha and beta particles respectively.
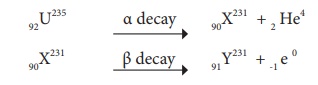
Number of neutrons = Mass number – Atomic number = 231 – 91 = 140
Solved problem 6.4
Calculate the amount of energy released when a radioactive substance undergoes fusion and results in a mass defect of 2 kg.
Solution:
Mass defect in the reaction (m) = 2 kg
Velocity of light (c) = 3 × 108 m s-1
By Einstein’s equation,
Energy released E = mc2
So E = 2 × (3 × 108)2
= 1.8 × 1017 J
Related Topics