Properties, Radioactive displacement law - Alpha, Beta and Gamma Rays | 10th Science : Chapter 6 : Nuclear Physics
Chapter: 10th Science : Chapter 6 : Nuclear Physics
Alpha, Beta and Gamma Rays
ALPHA, BETA AND GAMMA
RAYS
When a radioactive
nucleus undergoes radioactivity, it emits harmful radiations. These radiations
are usually comprised of any of the three types of particles. They are alpha(α),
beta (β) and gamma(γ) rays.
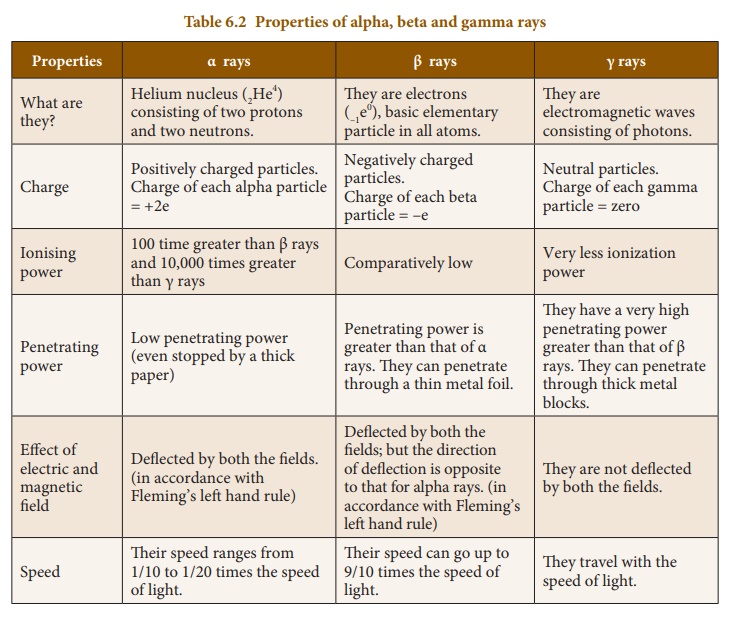
1. Properties of Alpha,
Beta and Gamma rays
These three particles
possess certain similarities and dissimilarities in their properties as listed
below in Table 6.2.
2. Radioactive displacement law
In 1913, Soddy and Fajan
framed the displacement laws governing the daughter nucleus produced during an
alpha and beta decay. They are stated below:
(i) When a radioactive
element emits an alpha particle, a daughter nucleus is formed whose mass number
is less by 4 units and the atomic number is less by 2 units, than the mass
number and atomic number of the parent nucleus.
(ii) When a radioactive element emits a beta particle, a daughter nucleus is formed whose mass number is the same and the atomic number is more by 1 unit, than the atomic number of the parent nucleus.
3. Alpha decay
A nuclear reaction in
which an unstable parent nucleus emits an alpha particle and forms a stable
daughter nucleus, is called 'alpha decay'.
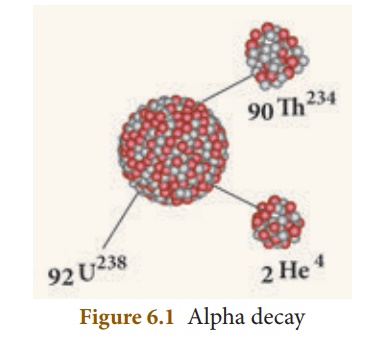
E.g.: Decay of uranium (U238)
to thorium (Th234) with the emission of an alpha particle.
92U238 → 90Th234 +2He4 ( α - decay )
In α - decay, the parent
nucleus emits an α particle and so it is clear that for the daughter nucleus,
the mass number decreases by four and the atomic number decreases by two as
illustrated in Figure 6.1
4. Beta decay
A nuclear reaction, in
which an unstable parent nucleus emits a beta particle and forms a stable
daughter nucleus, is called 'beta decay'.
E.g.: Beta decay of
phosphorous.
15P32 → 16S32
+ -1e0 (β - decay)
In β - decay there is no
change in the mass number of the daughter nucleus but the atomic number
increases by one.
Note: In a nuclear reaction,
the element formed as the product nucleus is identified by the atomic
number of the resulting nucleus and not by its mass number.
5. Gamma decay
In a γ - decay, only the
energy level of the nucleus changes. The atomic number and mass number of the
radioactive nucleus remain the same.
Related Topics