Discovery, Definition, Types, Units - Radioactivity | 10th Science : Chapter 6 : Nuclear Physics
Chapter: 10th Science : Chapter 6 : Nuclear Physics
Radioactivity
RADIOACTIVITY
1. Discovery of radioactivity
In 1896, French
physicist Henri Becquerel finished his research for the week and stored
a certain amount of uranium compound away in a drawer for the week end. By
chance, an unexposed photographic plate was also stored in the same drawer.
After a week he returned and noticed that the film had been exposed to some
radiation. He discovered that he could reproduce the effect whenever he placed
uranium near a photographic film. Apparently, uranium radiated something that
could affect a photographic plate. This phenomenon was called as Radioactivity.
Uranium was identified to be a radioactive element.
Two years later, the Polish physicist Marie Curie and her husband Pierre Curie detected radioactivity in 'Pitchblende', a tiny black substance. They were not surprised at the radioactivity of pitchblende, which is known as an ore of uranium. Later, they discovered that the radiation was more intense from pure uranium. Also, it was found that the pitchblende had less concentration of uranium. They concluded that some other substance was present in pitchblende. After separating this new substance, they discovered that it had unknown chemical properties and it also emitted radiations spontaneously like uranium. They named this new substance as 'Radium'.The radioactive elements emit harmful radioactive radiations like alpha rays or beta rays or gamma rays.
2. Definition of radioactivity
The nucleus of some
elements is unstable. Such nuclei undergo nuclear decay and get converted into
more stable nuclei. During this nuclear reaction, these nuclei emit certain
harmful radiations and elementary particles. The phenomenon of nuclear decay of
certain elements with the emission of radiations like alpha, beta, and gamma
rays is called 'radioactivity' and the elements, which undergo this phenomenon
are called 'radioactive elements'.

 3. Natural
Radioactivity
3. Natural
Radioactivity
The elements such as
uranium and radium undergo radioactivity and emit the radiations on their own
without any human intervention. This phenomenon of spontaneous emission of
radiation from certain elements on their own is called 'natural radioactivity'.
The elements whose
atomic number is more than 83 undergo spontaneous radioactivity. Eg: uranium,
radium, etc. There are only two elements, which have been identified as
radioactive substances with atomic number less than 83. They are technetium
(Tc) with atomic number 43 and promethium (Pm) with atomic number 61.
4. Artificial Radioactivity (or) Induced Radioactivity
The phenomenon by which
even light elements are made radioactive, by artificial or induced methods, is
called 'artificial radioactivity' or 'man-made radioactivity'.
This kind of radioactivity was discovered by Irene Curie and F.Joliot in 1934. Artificial radioactivity is induced in certain lighter elements like boron, aluminium etc., by bombarding them with radiations such as 'alpha particles' emitted during the natural radioactivity of uranium. This also results in the emission of invisible radiations and elementary particles. During such a disintegration, the nucleus which undergoes disintegration is called 'parent nucleus' and that which is produced after the disintegration is called a 'daughter nucleus'.
The particle, which is used to induce the
artificial disintegration is termed as projectile and the particle which is
produced after the disintegration is termed as ejected particle. When the
projectile hits the parent nucleus, it is converted into an unstable nucleus,
which in turn decays spontaneously emitting the daughter nucleus along with an
ejected particle.
If you denote the parent
and daughter nuclei as X and Y respectively, then the nuclear disintegration is
represented as follows: X (P,E) Y. Here, P and E represent the projectile
particle and ejected particle respectively.
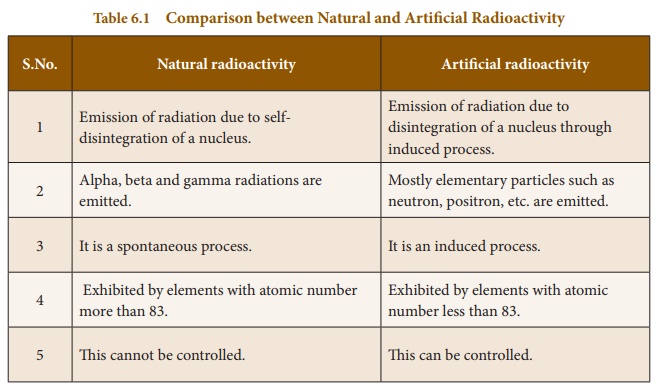
Comparison between Natural and Artificial Radioactivity
Natural radioactivity
1. Emission of radiation due to
self-disintegration of a nucleus.
2 Alpha, beta and gamma radiations
are emitted.
3 It is a spontaneous process.
4 Exhibited by elements with atomic
number more than 83.
5 This cannot be controlled.
Artificial radioactivity
Emission of radiation due to
disintegration of a nucleus through induced process.
Mostly elementary particles such as
neutron, positron, etc. are emitted.
It is an induced process.
Exhibited by elements with atomic
number less than 83.
This can be controlled.
Example:
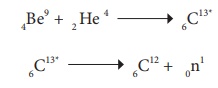
In the above nuclear
reaction, 6C13* is unstable and is radioactive. This
reaction can be represented as 4Be9 (α, n) 6C12

5. Units of Radioactivity
Curie: It is the traditional
unit of radioactivity. It is defined as the quantity of a radioactive
substance which undergoes 3.7 × 1010 disintegrations in one second.
This is actually close to the activity of 1 g of radium 226.
1 curie = 3.7 × 1010
disintegrations per second.
Rutherford (Rd): It is another unit of
radioactivity. It is defined as the quantity of a radioactive substance,
which produces 106 disintegrations in one second.
1 Rd = 106
disintegrations per second.
Becquerel (Bq) : It is The SI unit of radioactivity
is becquerel. It is defined as the quantity of one disintegration per second.
Roentgen (R): It is The radiation
exposure of γ and x-rays is measured by another unit called roentgen.
One roentgen is defined as the quantity of radioactive substance which produces
a charge of 2.58 × 10-4 coulomb in 1 kg of air under standard
conditions of pressure, temperature and humidity.
Related Topics