Definition, materials, Chain Reaction, Critical Mass - Nuclear Fission | 10th Science : Chapter 6 : Nuclear Physics
Chapter: 10th Science : Chapter 6 : Nuclear Physics
Nuclear Fission
NUCLEAR FISSION
1. Definition
In 1939, German Scientist
Otto Hahn and F.Strassman discovered that when a uranium nucleus is bombarded
with a neutron, it breaks up into two smaller nuclei of comparable mass along
with the emission of a few neutrons and energy. This process of breaking
(splitting) up of a heavier nucleus into two smaller nuclei with the release of
a large amount of energy and a few neutrons is called 'nuclear fission'.
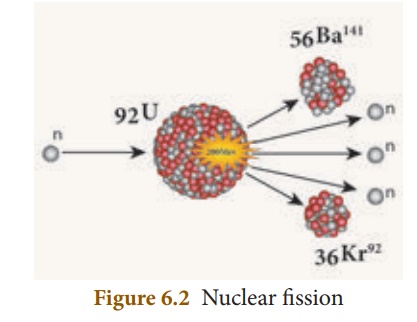
E.g.: Nuclear fission of a
uranium nucleus (U235)
92U235 + 0n1 → 56Ba141 + 36Kr92
+ 30n1 + Q (energy)
The average energy
released in each fission process is about 3.2 × 10 -11 J. Nuclear
fission is pictorially represented in Figure 6.2.
2. Fissionable materials
A fissionable material
is a radioactive element, which undergoes fission in a sustained manner when it
absorbs a neutron. It is also termed as 'fissile material'.
E.g.: U235,
plutonium (Pu239 and Pu241)
All isotopes of uranium
do not undergo nuclear fission when they absorb a neutron. For example, natural
uranium consists of
99.28 % of 92U238 and 0.72 % of
92U235. Of these two, U238 does not undergo
fission whereas U235 undergoes fission. Hence, U235 is a
fissionable material and U238 is non-fissionable.
There are some
radioactive elements, which can be converted into fissionable material. They
are called as fertile materials.
E.g.: Uranium-238,
Thorium-232, Plutonium-240.
3. Chain Reaction
A uranium nucleus
(U-235) when bombarded with a neutron undergoes fission producing three
neutrons. These three neutrons in turn can cause fission in three other uranium
nuclei present in the sample, thus producing nine neutrons. These nine neutrons
in turn may produce twenty seven neutrons and so on. This is known as 'chain
reaction'. A chain reaction is a self-propagating process in which the number
of neutrons goes on multiplying rapidly almost in a geometrical progression.
Two kinds of chain
reactions are possible. They are: (i) controlled chain reaction and (ii) uncontrolled
chain reaction.
(a) Controlled chain reaction
In the controlled chain
reaction the number of neutrons released is maintained to be one. This is
achieved by absorbing the extra neutrons with a neutron absorber leaving only
one neutron to produce further fission. Thus, the reaction is sustained in a
controlled manner. The energy released due to a controlled chain reaction can
be utilized for constructive purposes. Controlled chain reaction is used in a
nuclear reactor to produce energy in a sustained and controlled manner.
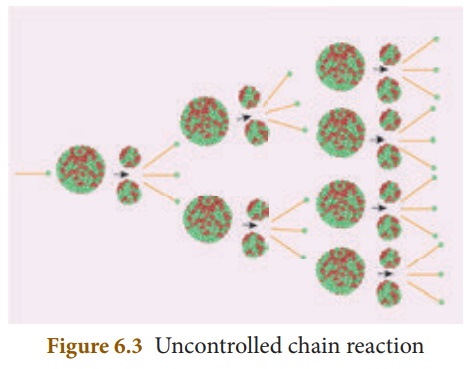
(b) Uncontrolled chain reaction
In the uncontrolled
chain reaction the number of neutrons multiplies indefinitely and causes
fission in a large amount of the fissile material. This results in the release
of a huge amount of energy within a fraction of a second. This kind of chain
reaction is used in the atom bomb to produce an explosion. Figure 6.3
represents an uncontrolled chain reaction.
4. Critical Mass
During a nuclear fission
process, about 2 to 3 neutrons are released. But, all these neutrons may not be
available to produce further fission. Some of them may escape from the system,
which is termed as 'leakage of neutrons' and some may be absorbed by the
non-fissionable materials present in the system. These two factors lead to the
loss of neutrons. To sustain the chain reaction, the rate of production of
neutrons due to nuclear fission must be more than the rate of its loss. This
can be achieved only when the size (i.e., mass) of the fissionable material is
equal to a certain optimum value. This is known as 'critical mass'.
The minimum mass of a
fissile material necessary to sustain the chain reaction is called 'critical
mass (mc)'. It depends on the nature, density and the size of the
fissile material.
If the mass of the
fissile material is less than the critical mass, it is termed as 'subcritical'.
If the mass of the fissile material is more than the critical mass, it is
termed as 'supercritical'.
5. Atom bomb
The atom bomb is based
on the principle of uncontrolled chain reaction. In an uncontrolled chain
reaction, the number of neutrons and the number of fission reactions multiply
almost in a geometrical progression. This releases a huge amount of energy in a
very small time interval and leads to an explosion.
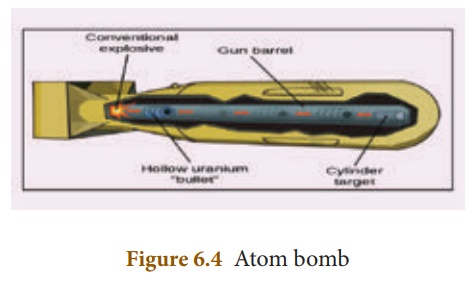
Structure:
An atom bomb consists of
a piece of fissile material whose mass is subcritical. This piece has a
cylindrical void. It has a cylindrical fissile material which can fit into this
void and its mass is also subcritical. When the bomb has to be exploded, this
cylinder is injected into the void using a conventional explosive. Now, the two
pieces of fissile material join to form the supercritical mass, which leads to
an explosion. The structure of an atom bomb is shown in Figure 6.4
During this explosion
tremendous amount of energy in the form of heat, light and radiation is
released. A region of very high temperature and pressure is formed in a
fraction of a second along with the emission of hazardous radiation like γ
rays, which adversely affect the living creatures. This type of atom bombs were
exploded in 1945 at Hiroshima and Nagasaki in Japan during the World War II.
Related Topics