Chapter: Medical Physiology: Rhythmical Excitation of the Heart
Sinus (Sinoatrial) Node
Sinus (Sinoatrial) Node
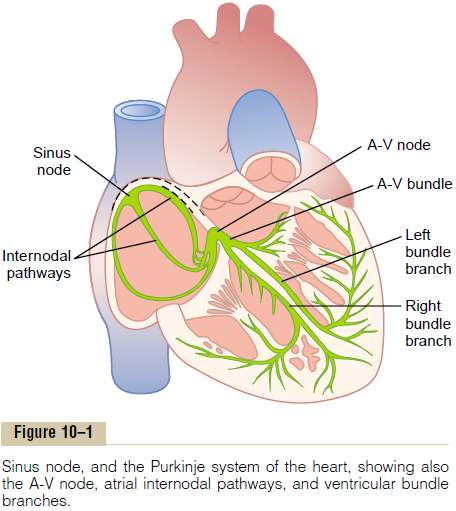
The sinus node (also called sinoatrial node) is a small, flattened, ellipsoid strip of specialized cardiac muscle about 3 millimeters wide, 15 millimeters long, and 1 millimeter thick. It is located in the superior posterolateral wall of the right atrium immediately below and slightly lateral to the opening of the superior vena cava. The fibers of this node have almost no contractile muscle filaments and are each only 3 to 5 micrometers in diameter, in contrast to a diameter of 10 to 15 micrometers for the surrounding atrial muscle fibers. However, the sinus nodal fibers connect directly with the atrial muscle fibers, so that any action potential that begins in the sinus node spreads immediately into the atrial muscle wall.
Automatic Electrical Rhythmicity of the Sinus Fibers
Some cardiac fibers have the capability of self-excitation, a process that can cause automatic rhythmical discharge and contraction. This is especially true of the fibers of the heart’s specialized conducting system, including the fibers of the sinus node. For this reason, the sinus node ordinarily controls the rate of beat of the entire heart. First, let us describe this automatic rhythmicity.
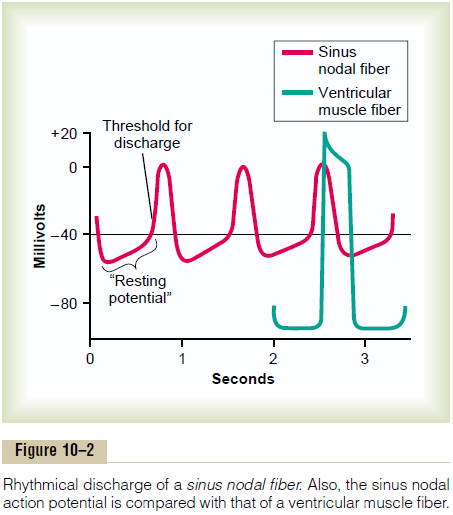
Mechanism of Sinus Nodal Rhythmicity. Figure 10–2 showsaction potentials recorded from inside a sinus nodal fiber for three heartbeats and, by comparison, a single ventricular muscle fiber action potential. Note that the “resting membrane potential” of the sinus nodal fiber between discharges has a negativity of about -55 to -60 millivolts, in comparison with -85 to -90 millivolts for the ventricular muscle fiber. The cause of this lesser negativity is that the cell membranes of the sinus fibers are naturally leaky to sodium and calcium ions, and positive charges of the entering sodium and calcium ions neutralize much of the intracellular negativity.
Before attempting to explain the rhythmicity of the sinus nodal fibers, first recall from the discussions that cardiac muscle has three types of membrane ion channels that play important roles in causing the voltage changes of the action potential. They are (1) fast sodium channels, (2) slow sodium-calcium channels, and (3) potassium channels. Openingof the fast sodium channels for a few 10,000ths of a second is responsible for the rapid upstroke spike of the action potential observed in ventricular muscle, because of rapid influx of positive sodium ions to the interior of the fiber. Then the “plateau” of the ven-tricular action potential is caused primarily by slower opening of the slow sodium-calcium channels, which lasts for about 0.3 second. Finally, opening of potas-sium channels allows diffusion of large amounts of positive potassium ions in the outward direction through the fiber membrane and returns the mem-brane potential to its resting level.
But there is a difference in the function of these channels in the sinus nodal fiber because the “resting” potential is much less negative—only -55 millivolts in the nodal fiber instead of the -90 millivolts in the ven-tricular muscle fiber. At this level of -55 millivolts, the fast sodium channels mainly have already become “inactivated,” which means that they have become blocked. The cause of this is that any time the mem-brane potential remains less negative than about -55 millivolts for more than a few milliseconds, the inacti-vation gates on the inside of the cell membrane that close the fast sodium channels become closed and remain so. Therefore, only the slow sodium-calcium channels can open (i.e., can become “activated”) and thereby cause the action potential. As a result, the atrial nodal action potential is slower to develop than the action potential of the ventricular muscle. Also, after the action potential does occur, return of the potential to its negative state occurs slowly as well, rather than the abrupt return that occurs for the ventricular fiber.
Self-Excitation of Sinus Nodal Fibers. Because of thehigh sodium ion concentration in the extracellular fluid outside the nodal fiber, as well as a moderate number of already open sodium channels, positive sodium ions from outside the fibers normally tend to leak to the inside. Therefore, between heartbeats, influx of positively charged sodium ions causes a slow rise in the resting membrane potential in the positive direction. Thus, as shown in Figure 10–2, the “resting” potential gradually rises between each two heartbeats. When the potential reaches a threshold voltage of about -40 millivolts, the sodium-calcium channels become “activated,” thus causing the action potential. Therefore, basically, the inherent leakiness of the sinus nodal fibers to sodium and calcium ions causes their self-excitation.
Why does this leakiness to sodium and calcium ions not cause the sinus nodal fibers to remain depolarized all the time? The answer is that two events occur during the course of the action potential to prevent this. First, the sodium-calcium channels become inac-tivated (i.e., they close) within about 100 to 150 mil-liseconds after opening, and second, at about the same time, greatly increased numbers of potassium channels open. Therefore, influx of positive calcium and sodium ions through the sodium-calcium channels ceases, while at the same time large quantities of positive potassium ions diffuse out of the fiber. Both of these effects reduce the intracellular potential back to its negative resting level and therefore terminate the action potential. Furthermore, the potassium channels remain open for another few tenths of a second, tem-porarily continuing movement of positive charges out of the cell, with resultant excess negativity inside the fiber; this is called hyperpolarization. The hyperpolar-ization state initially carries the “resting” membrane potential down to about -55 to -60 millivolts at the termination of the action potential.
Last, we must explain why this new state of hyper-polarization is not maintained forever. The reason is that during the next few tenths of a second after the action potential is over, progressively more and more potassium channels close. The inward-leaking sodium and calcium ions once again overbalance the outward flux of potassium ions, and this causes the “resting” potential to drift upward once more, finally reaching the threshold level for discharge at a potential of about -40 millivolts. Then the entire process begins again: self-excitation to cause the action potential, recovery from the action potential, hyperpolarization after the action potential is over, drift of the “resting” potential to threshold, and finally re-excitation to elicit another cycle. This process continues indefinitely throughout a person’s life.
Related Topics