Chapter: Clinical Dermatology: Diagnosis of skin disorders
Side-room and office tests - Diagnosis of skin disorders
Side-room and office tests
A
number of tests can be performed in the practice office so that their results
will be available immediately.
Potassium hydroxide preparations for fungal infections
If
a fungal infection is suspected, scales or plucked hairs can be dissolved in an
aqueous solution of 20% potassium hydroxide (KOH) containing 40% dimethyl
sulphoxide (DMSO). The scale from the edge of a scaling lesion is vigorously
scraped on to a glass slide with a No. 15 scalpel blade or the edge of a second
glass slide. Other samples can include nail clippings, the roofs of blisters,
hair pluckings, and the contents of pustules when a candidal infection is
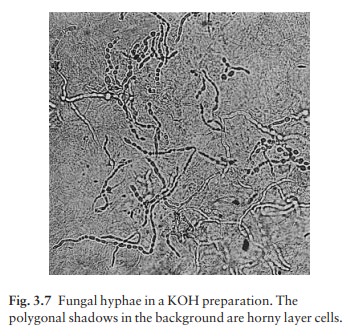
suspected. A drop or two of the KOH solution is run under the cover slip (Fig.
3.6). After 5ŌĆō10 min the mount is examined under a microscope with the
condenser lens lowered to increase contrast. Nail clippings take longer to
clearaup to a couple of hours. With experience, fungal and candidal hyphae can
be readily detected (Fig. 3.7). No heat is required if DMSO is included in the
KOH solution.

Detection of a scabies mite
Burrows
in an itchy patient are diagnostic of scabies. Retrieving a mite from the skin
will confirm the diagnosis and convince a sceptical patient of the infestation.
The burrow should be examined under a magnifying glass; the acarus is seen as a
tiny black or grey dot at the most recent, least scaly end. It can
Alternatively, if mites are not seen, possible burrows can be vigorously
scraped with a No. 15 scalpel blade, moistened with liquid paraffin or
vegetable oil, and the scrapings transferred to a slide. Patients never argue
the toss when confronted by a magnified mobile mite. Dermatoscopy can also be used to detect the scabies mite.
Cytology (Tzanck smear)
Cytology
can aid diagnosis of viral infections such as herpes simplex and zoster, and of
bullous diseases such as pemphigus. A blister roof is removed and the cells
from the base of the blister are scraped off with a No. 10 or 15 surgical
blade. These cells are smeared on to a microscope slide, air-dried and fixed
with methanol. They are then stained with Giemsa, toluidine blue or WrightŌĆÖs
stain. Acantholytic cells are seen in
pemphigus and multinucleate giant cells are dia-gnostic of herpes simplex or
varicella zoster infec-tions . Practice is needed to get good preparations. The
technique remains popular in the USA but has fallen out of favour in the UK as
his-tology, virological culture and electron microscopy have become more
accessible.
Patch tests
Patch
tests are invaluable in detecting the allergens responsible for allergic
contact dermatitis .
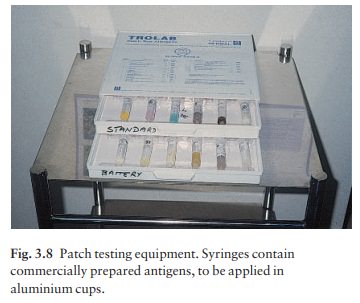
Either
suspected individual antigens, or a battery of antigens which are common
culprits, can be tested. Standard dilutions of the common antigens in
appro-priate bases are available commercially (Fig. 3.8). The test materials
are applied to the back under aluminium discs or patches; the occlusion
encourages penetration of the allergen. The patches are left in place for 48 h
and then, after careful marking, are removed. The sites are inspected 10 min
later, again at 96 h and some-times even later if doubtful reactions require
further assessment. The test detects type IV delayed hyper-sensitivity
reactions . The readings are scored according to the reaction seen. NT Not
tested.
0 No reaction.
┬▒ Doubtful
reaction (minimal erythema).
+ Weak
reaction (erythematous and maybe papular).
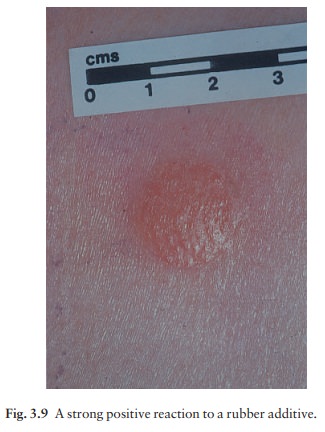
++ Strong
reaction (erythematous and oedematous or vesicular; Fig. 3.9).
++ + Extreme
reaction (erythematous and bullous). IR Irritant reaction (variable, but often
sharply cir-cumscribed, with a glazed appearance and increased skin markings).
A
positive patch test does not prove that the allergen in question has caused the
current episode of contact dermatitis; the results must be interpreted in the
light of the history and possible previous exposure to the allergen.
Patch
testing requires attention to detail in applying the patches properly, and
skill and experience in inter-preting the results.
Prick testing
Prick
testing is much less helpful in dermatology. It detects immediate (type I)
hypersensitivity and patients should not
have taken systemic antihis-tamines for at least 48 h before the test.
Commercially prepared diluted antigens and a control are placed as single drops
on marked areas of the forearm. The skin is gently pricked through the drops
using separate sterile fine (e.g. Size 25 gauge, or smaller) needles. The prick
should not cause bleeding. The drops are then removed with a tissue wipe. After
10 min the sites are inspected and the diameter of any wheal measured and
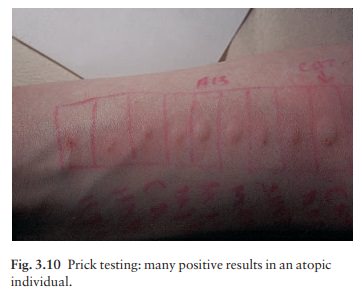
recorded. A result is considered positive if the test antigen causes a wheal of
4 mm or greater (Fig. 3.10) and the control elicits negligible reaction. Like
patch testing, prick testing should not be undertaken by those without formal
training in the procedure. Although the risk of anaphylaxis is small,
resuscitation facilities including adrenaline (epinephrine) and oxygen must be available. The relevance of positive
results to the cause of the condition under investigationausually urticaria or
atopic dermatitisais often debatable. Positive results should correlate with
positive radio-allergosorbent tests (RAST;) used to measure total and specific
immunoglobulin E (IgE) levels to
Skin biopsy
Biopsy
(from the Greek bios
meaning ŌĆślifeŌĆÖ and opsis ŌĆśsightŌĆÖ) of skin lesions is useful
to establish or con-firm a clinical diagnosis. A piece of tissue is removed
surgically for histological examination and, sometimes, for other tests (e.g.
culture for organisms). When used selectively, a skin biopsy can solve the most
perplexing problem but, conversely, will be unhelpful in con-ditions without a
specific histology (e.g. most drug eruptions, pityriasis rosea, reactive
erythemas).
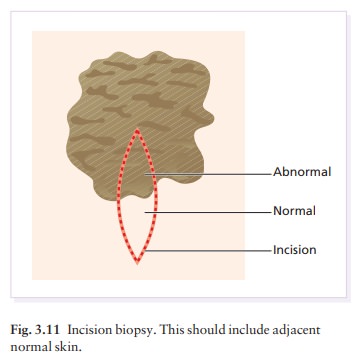
Skin
biopsies may be incisional,
when just part of a lesion is removed for laboratory examination or excisional,
when the whole lesion is cut out. Exci-sional biopsy is preferable for most
small lesions (up to 0.5 cm diameter) but incisional biopsy is chosen when the
partial removal of a larger lesion is adequate for diagnosis, and complete
removal might leave an unnecessary and unsightly scar. Ideally, an incisional
biopsy should include a piece of the surrounding normal skin (Fig. 3.11)
although this may not be possible if a small punch is used.
The
main steps in skin biopsy are:
administration of local anaesthesia;
and
removal of all (excision) or part
(incision) of the lesion and repair of the defect made by a scalpel or punch.
Local anaesthetic
Lignocaine
(lidocaine) 1ŌĆō2% is used. Sometimes adrena-line 1 : 200 000 is added. This
causes vasoconstriction, reduced clearance of the local anaesthetic and
pro-longation of the local anaesthetic effect. Plain lignocaine should be used
on the fingers, toes and penis as the prolonged vasoconstriction produced by
adrenaline can be dangerous here. Adrenaline is also best avoided in diabetics
with small vessel disease, in those with a history of heart disease (including
dysrhythmias), in patients taking non-selective ╬▒
blockers and tricyclic antidepressants (because of potential interactions) and
in uncontrolled hyperthyroidism. There are exceptions to these general rules
and, undoubtedly, the total dose of local anaesthetic and/or adrenaline is
important. Nevertheless, the rules should not be broken unless the surgeon is
quite sure that the procedure that he or she is about to embark on is safe.
It
is wise to avoid local anaesthesia during early pregnancy and to delay
non-urgent procedures until after the first trimester.
As
ŌĆśBŌĆÖ follows ŌĆśAŌĆÖ in the alphabet, get into the habit of checking the precise
concentration of the lignocaine added adrenaline on the label before
withdrawing it into the syringe and then, before injecting
it, confirm that the patient has not had any previous allergic reac-tions to
local anaesthetic.
Infiltration
of the local anaesthetic into the skin around the area to be biopsied is the
most widely used method. If the local anaesthetic is injected into the
subcutaneous fat, it will be relatively pain-free, will produce a diffuse
swelling of the skin and will take several minutes to induce anaesthesia.
Intradermal injections are painful and produce a discrete wheal associated with
rapid anaesthesia. The application of EMLA cream (eutectic mixture of local
anaesthesia) to the operation site 2 h before giving a local anaesthetic to
children helps to numb the initial prick.
Scalpel biopsy
This
provides more tissue than a punch biopsy. It can be used routinely, but is
especially useful for biopsy-ing disorders of the subcutaneous fat, for
obtaining specimens with both normal and abnormal skin for comparison (Fig.
3.11) and for removing small lesions in toto (excision biopsy,). After selecting
thelesion for biopsy, an elliptical piece of skin is excised.

The
specimen should include the subcutaneous fat. Removing the specimen with
forceps may cause crush artefact, which can be avoided by lifting the specimen
with either a Gillies hook or a syringe needle. The wound is then sutured; firm
compression for 5 min stops oozing. Non-absorbable 3/0 sutures are used for
biopsies on the legs and back, 5/0 for the face, and 4/0 for elsewhere.
Stitches are usually removed from the face in 4 days, from the anterior trunk
and arms in 7 days, and from the back and legs in 10 days. Some guidelines for
skin biopsies are listed in Table 3.3.
Punch biopsy
The
skin is sampled with a small (3 ŌĆō 4 mm diameter) tissue punch. Lignocaine 1% is
injected intradermally
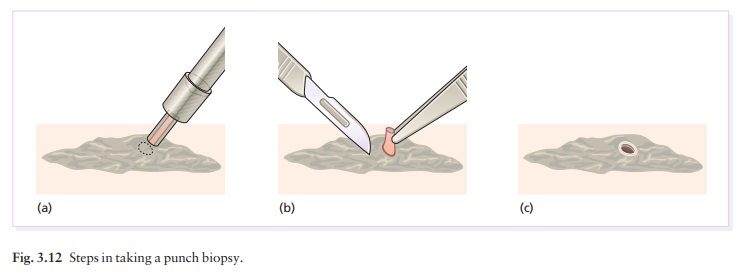
Skin is lifted up carefully with a needle or forceps and the base
is cut off at the level of subcutaneous fat. The defect is cauterized or
repaired with a single suture. The biopsy specimen must not be crushed with the
forceps or critical histological patterns may be distorted.
The
tissue can be sent to the pathologist with a summary of the history, a
differential diagnosis and the patientŌĆÖs age. Close liaison with the
pathologist is essential, because the diagnosis may only become apparent with
knowledge of both the clinical and his-tological features.
Related Topics