Chapter: Modern Medical Toxicology: Miscellaneous Drugs and Poisons: Analgesics and Antihistamines
Salicylates - Analgesic-Antipyretics
ANALGESIC-ANTIPYRETICS
The
analgesic -antipyretics of paramount importance are salicylates and
paracetamol. Phenacetin is no more used today. Analgin has serious adverse
effects but is still available in India. Nefopam is a new entrant.
Salicylates
These
compounds are derivatives of salicylic acid and include acetyl salicylic acid,
sodium salicylate, and methyl salicylate. Salicin, a naturally occurring
salicylate is a constituent of several plants but is present in highest
concentration in the willow tree (Salix
alba vulgaris) (Fig 29.1), which
grows near lakes and rivers in temperate climates and whose branches are used
to make cricket bats and baskets. Other plants include Acacia (flower oil),
Aspens, Birches, Calycanthus (leaves), Camellia (leaves), Chenopodium (leaves),
Hyacinth, Marigold, Milkwort, Poplars, Spiraea, Teaberry, Tulips and Violets.

Salicylic
acid (orthohydroxy benzoic acid) is so irritating that it can only be used as
an external application. Hoffman, a
chemist at Bayer Company first synthesised acetyl salicylic acid in the
laboratory in 1897, and together
with his chief pharmacologist Heinrich
Dreser, he performed a number of pharmacological and toxicological tests to
evaluate its therapeutic benefits and safety profile. The name “aspirin” was
coined in 1899, and since then the
drug has marched through time displaying a rare and astonishing staying power.
In fact, in recent times aspirin has surprised the medical profession with
newer and far more exciting applications (vide
infra).
Examples
Acetaminosalol,
Aloxiprin, Aluminium aspirin, Ammonium salicylate, Antipyrine salicylate,
Aspirin, Benorylate, Bismuth subsalicylate, Bromosalicylic acid acetate,
Calcium aminosal-icylate, Calcium carbaspirin, Carbamoylphenoxyacetic acid,
Choline salicylate, Diethylamine salicylate, Ethyl salicylate, Fendosal, Glycol
salicylate, Homomenthyl salicylate, Lithium salicylate, Magnesium salicylate,
Menthyl salicylate, Octyl salicylate, Phenazone salicylate, Phenyl
aminosalicylate, Phenyl salicylate, Physostigmine salicylate, Potassium
aminosalicylate, Potassium salicylate, Salicylamide, Salicylic acid, Salsalate,
Silver salicylate, Sodium aminosalicylate, Sodium salicylate, Sodium
thiosalicylate, Thurfyl salicylate, Triethanolamine salicylate, Trolamine salicylate
Physical Appearance
Acetyl
salicylic acid is an odourless, white, crystalline powder with an unpleasant
saline taste. Sodium salicylate occurs as odourless, white scaly crystals with
the same unpleasant saline taste. Methyl salicylate is a colourless liquid with
aromatic odour and sweetish taste.
Salicylates
for therapeutic use are available as tablets, capsules, powders, effervescent
tablets and liquid preparations for ingestion; rectal suppositories; and as
liniments, creams and lotions for topical application.
Uses
Sodium salicylate and acetyl salicylic acid:
·
Antipyretic.
·
Analgesic.
·
Treatment of rheumatoid arthritis.
·
Low-dose aspirin is used in the prophylaxis of
cere-brovascular ischaemic events, angina pectoris, and is also recommended by
some authorities for the preven-tion of colon cancer, and migraine.
Accumulating data suggests that aspirin may prevent or protect against the
development of colon and possibly other types of gastrointestinal cancers.
Combination of warfarin with aspirin may improve efficacy in preventing
ischaemic heart disease, but may ironically increase the chances of a
haemorrhagic fatal stroke.
Sodium aminosalicylate:
·
It is used sometimes as a
second-line drug in the management of tuberculosis.
Bismuth subsalicylate:
·
It is used to treat diarrhoea, and
as prophylaxis for travellers diarrhoea.
New derivatives of salicylic acid:
·
Mesalamine (5-aminosalicylic acid)
is used as a suppos-itory or rectal suspension enema for its local effects in
the treatment of inflammatory bowel disease (proctosig-moiditis). Olsalazine
(sodium azodisalicylate) is said to be effective in relieving manifestations of
ulcerative colitis, and can be administered orally. Sulfasalazine
(salicylazosulfapyridine) is also beneficial.
·
Diflunisal, a difluorophenyl
derivative of salicylic acid is said to be much more potent than aspirin in the
treat-ment of musculoskeletal sprains and osteoarthritis.
·
Benorylate
(4-acetamidophenyl-o-acetylsalicylate) is an ester of aspirin and paracetamol.
It causes less gastric irritation and bleeding. The usual therapeutic dose in
adults is 4 gm/day. Toxicity can result if this is exceeded. Manifestations are
a combination of those seen in aspirin and paracetamol poisoning with
partic-ular tendency toward centrilobular hepatic necrosis.
Locally acting salicylates:
·
Salicylic acid is a keratolytic agent.
·
Methyl salicylate (oil
of wintergreen, sweet birch oil,gautheria oil), is used for the local
treatment of musculo-skeletal pain and inflammation. Commercial preparations
are not less than 98% w/w. One ml of 98% methyl salicy-late is equivalent to
1.4 grams ASA in salicylate potency, and its action is the same as salicylates;
or one teaspoonful of oil of wintergreen (5 ml) is equivalent to approximately
7000 mg of salicylate or 21.7 adult aspirin tablets.
·
Methyl salicylate is also used as a flavouring agent for
candy.
·
Homomenthyl salicylate (homosalate) is a sunscreen agent
found in many sunscreen products and contains 46% salicylic acid. Homosalate
could be hydrolysed in vivo to free salicylic acid and homomenthol.
·
Trolamine salicylate cream (10 grams of cream contains 500
mg of salicylic acid) is used in the management of osteoarthritis.
·
Salicylates are often combined with antihistamines and
decongestants, or caffeine in cold and allergy preparations. Several products
contain combinations of paracetamol and salicylate, while
others combine salicylate with opioids.![]()
Toxicokinetics
Salicylates
are rapidly absorbed from the stomach, and to a slightly lesser extent from the
small intestine. Delayed absorption is seen in the following situations:
enteric coated preparations, pylorospasm, pyloric stenosis, and bezoar
formation. Therapeutic serum salicylate levels should not exceed 30 mg/100 ml.
Salicylic acid and
methyl salicylate are readily absorbed through intact skin. Salicylates
distribute well into plasma; saliva; milk; and spinal, peritoneal and synovial
fluid and into body tissues including kidney, liver, lung and heart.
Metabolism occurs chiefly in the
liver, where salicylates are broken down into salicyluric acid, ether
glucoronide, ester glucoronide, and gentisic acid. Excretion is mainly through
urine.
The half-life of salicylates is 2 to
4 hours at therapeutic levels, but may increase to 20 hours at toxic levels.
Plasma salicylate is 50 to 80% protein bound, especially to albumin, with
salicylic acid being more highly bound than aspirin. As salicylate doses are
increased, the proportion bound to plasma protein decreases, and the volume of
distribution increases. There is also a decrease in protein binding from 90% at
thera-peutic levels to less than 75% at toxic levels. The apparent volume of
distribution increases from 0.2 L/kg to more than 0.3 L/kg. The half-life of
plasma salicylate elimination increases with dose. The reported half-lives in
adults range from 2.4 to 19 hours with doses of 0.25 gram, and about 10 to 20
grams of sodium salicylate, respectively. In poisoned children, the half-life
ranged from 15 to 29 hours.
Sustained release preparations of
aspirin contain aspirin released over a 12-hour or longer period of time.
Prolonged absorption and persistently elevated salicylate levels may occur
following overdose. Enteric-coated formulations are designed to dissolve in the
alkaline medium of the small intestine, and are likely to cause bezoars and
prolonged drug absorption.
Mode of Action
■■Salicylates stimulate the respiratory centre in the brainstem leading to hyperventilation and respiratory alkalosis. They also interfere with Krebs cycle, inhibit production of ATP, and increase lactate production, leading to ketosis and a wide anion-gap metabolic acidosis. In children, respira-tory alkalosis is quite transient, and metabolic acidosis is the predominant feature. Respiratory acidosis in salicylate overdose indicates grave prognosis and is seen in salicylate-induced pulmonary oedema, CNS depression from mixed overdose,* or severe fatigue due to prolonged hyperven-tilation.
■■ Salicylates
are extremely irritating to the GI mucosa, and overdose often results in
haemorrhagic gastritis. In the US, the FDA requires an alcohol warning on all
over-the-counter pain relievers, which includes aspirin, other salicylates,
paracetamol, ibuprofen, ketoprofen, and naproxen sodium, due to a potential
drug interaction resulting in upper GI bleed or liver damage.
■■ Aspirin
is commonly involved in allergic reactions, ranging in severity from urticaria
or angioedema to acute anaphy-laxis.
Drug Interactions
■■ Salicylate
and/or acetazolamide toxicity may occur in patients taking salicylates
chronically when acetazolamide is added to drug regimen. The syndrome of
effects reported are confusion, fatigue, hyperchloraemic metabolic acidosis,
incontinence, lethargy, and somnolence shortly after the introduction of
acetazolamide in patients chronically receiving aspirin.
■■ Effective
October, 1998, the US FDA mandated that products containing aspirin or other
salicylates display the following warning regarding chronic consumption of
alcohol and salicylate use, “Alcohol warning: If you consume 3 or more
alcoholic drinks every day, ask your doctor whether you should take this
medication or other pain relievers/fever reducers. This medication may cause stomach
bleeding”.
Clinical (Toxic) Features
Acute Poisoning:
·
Early—Nausea, vomiting, sweating,
tinnitus (ringingor hissing), vertigo, and hyperventilation due to respi-ratory
alkalosis. Irritability, confusion, disorientation, hyperactivity, slurred
speech, agitation, combativeness, hallucinations, ataxia, and restlessness may
be early findings in patients with severe toxicity.
·
Late—Deafness, hyperactivity, agitation,
delirium,convulsions, hallucinations, hyperpyrexia. Coma is unusual.
· Complications—Metabolic acidosis, pulmonaryoedema, rhabdomyolysis, cardiac depression, thrombo-cytopenic purpura. Gastrointestinal bleeding, perfora-tion and pancreatitis are less common complications. Salicylates must not be therapeutically administered to children under 15 years of age, especially if they are suffering from chicken pox or influenza. There is a serious risk of precipitating Reye’s syndrome which can be fatal (Table 29.1).
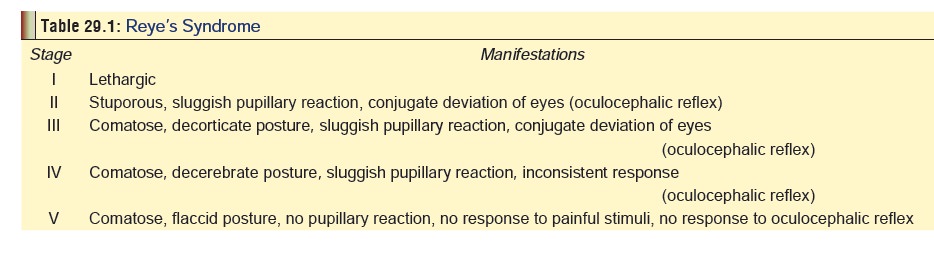
The main features are acute onset of hepatic failure and
encephalopathy. It prob-ably results from damage to mitochondria in liver
cells. Patients with Reye’s syndrome generally have elevated serum ammonia
levels, elevated LFT’s and an absent or low CSF salicylate level, while
salicylate intoxicated patients have higher serum and CSF salicylate levels.
Recovery is associated usually with permanent neuro-logical sequelae.
·
Respiratory alkalosis develops early in the course of
intoxication and may be the only acid base distur-bance with mild salicylism.
Respiratory alkalosis with compensatory metabolic acidosis develops in most
adults with moderate intoxication. Metabolic acidosis with acidaemia and
compensatory respiratory alkalosis develops in severe overdose and is
associated with a higher rate of complications and death.
·
The three most common auditory alterations described by
individuals after large doses of salicylates include tinnitus, loss of absolute
acoustic sensitivity, and alterations of perceived sounds. Symptoms can occur
gradually within the initial few days of therapy or within hours of an
extremely large dose.
·
Dehydration and hypokalaemia are common. QT prolongation, U
waves and flattened T waves have been described in several patients with
hypokalaemia after acute salicylate overdose.
·
Significant toxicity has been reported after chronic topical
use of creams and ointments containing salicy-lates. Salicylic acid found in
topical wart removal products at concentrations up to 17% (w/w) can cause
mucosal burns if ingested.
Chronic Poisoning (Salicylism):
·
This is characterised by slow onset
of confusion, agita-tion, lethargy, disorientation, slurred speech,
halluci-nations, convulsions, and coma. There may also be tinnitus, hearing
loss, nausea, dyspnoea, tachycardia and fever.
·
Sometimes “salicylism” presents as pseudosepsissyndrome characterised by
fever, leukocytosis, hypo-tension, and multi-organ system failure: ARDS, acute
renal failure and coagulopathy (DIC).
·
Prolongation of PT and PTT,
thrombocytopenia, hypofibrinogenaemia, elevation of fibrin degradation
products, and red blood cell fragmentation has devel-oped in some patients with
multiorgan system failure associated with chronic salicylate toxicity.
·
Chronic maternal ingestion is
associated with an increased incidence of stillbirths, antepartum/post-partum
bleeding, prolonged pregnancy/labour, and lower birth weight. The American
Academy of Pediatrics recommends that salicylates should be used cautiously
during breastfeeding; some studies also suggest that bismuth subsalicylates
consumed during lactation can lead to problems.
Treatment
·
In patients with severe poisoning,
examine the urine for calcium oxalate crystals. Also, monitor calcium and renal
function (BUN, creatinine).
·
Local treatment with cold milk or
ice cream as a demulcent is sufficient in most cases. Cold water or sucking on
crushed ice will also relieve oral pain. Remove all visible evidence of plant
debris from the oropharynx.
·
In severe cases, parenteral opioids,
corticosteroids, IV fluids, and endotracheal intubation may be required. Tetany
should be treated with intravenous calcium gluconate.
·
Ocular exposure to sap resulting in
chemical conjuncti- vitis and corneal abrasions must be treated with copious
irrigation, systemic analgesics, and expert ophthalmologic consultation.
Diagnosis
·
Monitor serum salicylate level,
glucose and electrolytes every 2 hours until the salicylate level is
consistently falling and acid base abnormalities are improving. Peak salicylate
may be delayed 6 hours or more following ingestion of tablets, and more than 12
hours after ingestion of enteric coated or sustained release products. Obtain
an arterial blood gas in symptomatic patients and follow until acid base
abnormalities are improving.
·
Obtain a CBC, renal and hepatic
function tests and INR or PT and PTT in patients with clinical evidence of
moderate to severe toxicity.
·
In patients with pyloric stenosis,
enteric coated aspirin has been shown to remain in the stomach for prolonged
periods of time. This can be shown by instillation of contrast media into the
stomach followed by an abdominal X-ray. This procedure should be considered in
patients with serum salicylate levels that do not decline or continue to rise.
Concretions of bismuth subsalicylate or enteric coated aspirin may be radiopaque
on plain abdominal radiographs.
Laboratory Findings:
·
Anion-gap acidosis.
·
Hypokalaemia (acidosis may mask it).
·
Hypocalcaemia.
·
Hypoglycaemia.
Bed-side Tests:
Ferric chloride test—
–– Add a few drops of 10% ferric
chloride solution to 1 ml of urine. A purple colour indicates the presence of
salicylates.
–– However it is not conclusive,
since a positive result is also obtained in phenol, phenothiazines,
phenyl-butazone, and oxyphenbutazone.
–– A method using ferric chloride on
methanolic extract of haemolysed whole blood has been described. The minimum
salicylate level this method can detect is 5 mg/100 ml.
Trinder’s test—
–– Reagent: Trinder’s reagent is
used which is obtained by mixing 40 grams of mercuric chloride (dissolved in
850 ml of purified water), with 120 ml of aqueous HCl (1 mol/L) and 40 grams of
hydrated ferric nitrate, followed by dilution to 1 litre with purified water.
––
Method: The test can be done on urine, stomach contents, or scene
residue. Add 0.1 ml of Trinder’s reagent to 2 ml of sample and mix for 5
seconds.
A strong violet colour indicates the
presence of salicylates. Mere darkening is not significant. If the sample to be
tested is stomach contents or scene residue, it is better to first boil 1 ml of
the sample with 1 ml of aqueous HCl (0.1 mol/L) for 10 minutes, cool, filter,
and then neutralise with 1 ml of aqueous sodium hydoxide (0.1 mol/L).
Confirmatory test—
––
The only confirmatory test is to estimate the serum salicylate level.
Unfortunately, the seriousness of poisoning correlates poorly with serum
levels. Previously, the Done nomogram (first published in 1960) was highly recommended to
correlate serum salicylate level with the degree of intoxica-tion at varying
intervals after acute ingestion of aspirin. But there are severe limitations to
its use and is now not generally considered to be reliable.
It has been shown to underestimate
or overestimate toxicity after salicylate ingestion, and is of no use in
evaluating toxicity after ingestion of enteric coated or sustained release
products, or in patients with subacute or chronic salicylism. Studies have
indicated that it has poor predictive value.
–– Whenever a serum salicylate level is obtained, it is essential to determine the concurrent arterial blood pH, since in the presence of acidaemia more salicylic acid leaves the blood and enters the CSF and other tissues, with consequent worsening of symptoms. Therefore, a falling serum salicylate level may be difficult to interpret as it can reflect either an increased tissue distribution with increased toxicity, or an increased clearance with decreased toxicity. A falling serum salicylate level accompanied by a falling or low blood pH should be presumed to reflect a serious or worsening situation, not a benign or improving one.
Treatment
·
Patients with major signs or symptoms (metabolic acidosis,
dehydration, mental status changes, seizures, pulmonary oedema) should be
admitted to the Intensive Care Unit regardless of serum salicylate level.
Patients with minor ![]() symptoms only (i.e. nausea,
tinnitus) following acute overdose may be managed in the emergency department
with decontamination and alkaline diuresis if the salicy-late level is shown to
be declining. Admission should be strongly considered regardless of the
salicylate level or symptoms in infants, children less than 2, the elderly, in
chronic overdose, or when the ingested tablets are enteric coated or sustained
release.
symptoms only (i.e. nausea,
tinnitus) following acute overdose may be managed in the emergency department
with decontamination and alkaline diuresis if the salicy-late level is shown to
be declining. Admission should be strongly considered regardless of the
salicylate level or symptoms in infants, children less than 2, the elderly, in
chronic overdose, or when the ingested tablets are enteric coated or sustained
release.
·
Stomach wash may be beneficial upto 12 hours after
ingestion, since toxic doses of salicylates often cause pylorospasm and delayed
gastric emptying. Whole bowel irrigation might be useful in patients with
bezoars, or patients who have ingested enteric coated or sustained release
products.
·
Activated charcoal (AC): It is said to be very efficacious
in the treatment of salicylate poisoning since each gram of AC can adsorb 550
mg of the drug. A 10:1 ratio of AC to salicylate ingested appears to result in
maximum efficiency. The initial dose of AC can be combined with a cathartic to
enhance elimination. Some investigators recommend multiple dosing of AC (i.e.
MDAC), while others do not consider it to be more beneficial.
·
Urinary alkalinisation: This should not be confused with forced diuresis which was recommended in
the past, wherethe accent was on increasing urinary flow rate in order to
increase salicylate clearance. It carries with it the risk of fluid overload
with attendant complications. Alkalinisation of both blood and urine can be
achieved with intravenous sodium bicarbonate.* Acetazolamide must not be used
since it produces a systemic metabolic acidosis.
·
Dose of NaHCO3 –
––
For mild poisoning: 1 mEq/kg of NaHCO3 is added to the first
bottle of 5% dextrose. If alkalinisation (i.e. urinary pH between 7.5 and 8.5)
is not achieved in a few hours, it can be repeated.
––
For severe poisoning: Additional bolus therapy of 50 to 100 mEq of NaHCO3
over 1 to 2 hours may be necessary.
–– Monitor serum electrolytes and
urine pH every 1 to 2 hours. Adjust potassium and bicarbonate administration as
needed to maintain a urine pH of 7.5 to 8. It is important to correct
hypokalaemia while alkalinising the urine. Alkalinisation should be stopped
when serum salicylate level falls below 35 mg/100 ml.
Haemodialysis: It is very effective
in salicylate poisoning and must always be considered in the presence of
cardiac or renal failure, intractable acidosis, convulsions, severe fluid
imbalance, or a serum salicylate level more than 100 mg/100 ml. Patients with
evidence of cerebral oedema require immediate dialysis. Charcoal haemoperfusion
produces better salicylate clearance than haemodialysis, but does not correct
fluid and electrolyte balance like haemodialysis.
·
Supportive measures:
o Correction of fluid and electrolyte
imbalance (watchout for fluid overload!).
o Correct dehydration with 0.9% saline
10 to 20 ml/kg/ hr over 1 to 2 hours until a good urine flow is obtained (at
least 3 to 6 ml/kg/hr). In patients, in whom urinary alkalinisation is being
considered, initial hydration may be with 10 to 20 ml/kg of D5W with 88 to 132
milliequivalents of bicarbonate added. Patients in shock may require more rapid
fluid administration.
o Hypoprothrombinaemia can be
corrected by 2.5 to 5 mg of vitamin K IV every day.
o Hyperpyrexia must be tackled by
cooling measures (e.g. ice in the axilla and groin).
o Correction of metabolic acidosis
with NaHCO3.
o Correction of hypocalcaemia with
calcium gluconate IV (5 to 10 ml in adults).
o Correct hypokalaemia as needed.
Patients undergoing forced or alkaline diuresis may require large amounts of
potassium supplementation due to renal potassium wasting. Institute continuous
cardiac monitoring in patients with hypokalaemia, and those requiring high
doses of potassium.
o Correction of hypoglycaemia with
glucose IV (50 ml of 5% dextrose or 1 ml/kg).
o Treatment of convulsions with benzodiazepines.
o Mild cerebral oedema and elevated
intracranial pres-sure (ICP) can be managed by head elevation and
administration of mannitol; hyperventilation should be performed if there is
evidence of impending herniation. Haemodialysis may be necessary.
o Salicylates can interfere with
coagulation mechanisms, therefore, patients with evidence of active bleeding or
coagulation disorders require laboratory monitoring to include prothrombin time
(PT) and INR. Give blood or blood products (fresh frozen plasma) if bleeding is
excessive. Vitamin K may be beneficial in the presence of a prolonged PT or
INR.
·
Treatment of Reye’s syndrome:
o Admit the patient to an intensive
care unit.
o Raise the head-end of bed (400).
o Mannitol IV (0.2 to 1.0 gm/kg).
o Acute hyperventilation may be
helpful.
o Short acting barbiturates in
resistant cases.
Autopsy Features
·
Petechiae in the skin
(occasionally).
·
Erosions of gastric mucosa. Black,
altered blood may lie in the stomach. Sometimes massed concretions of tablets
are present.
·
Petechiae in various organs and
serous membranes (parietal pleura, pericardium).
·
Pulmonary and cerebral oedema.
Forensic Issues
·
Most of the cases of overdose are
suicidal in nature; a few may be accidental.
·
In some individuals, a small dose of
aspirin can provoke a fatal hypersensitivity reaction. The patient is usually
(curi- ously) a middle-aged female, and often has nasal polyps. Within minutes
of ingestion there is an acute vasomotor rhinitis, angioneurotic oedema, and
urticaria. Death results from laryngeal oedema, hypotension, or shock.
·
Salicylate poisoning can also result
from extensive applica-tion of salicylate-containing ointments, keratolytic
agents, or other agents containing methyl salicylate.
Related Topics