Chapter: Modern Medical Toxicology: Miscellaneous Drugs and Poisons: Analgesics and Antihistamines
Paracetamol: Diagnosis, Treatment on Poisons - Analgesic-Antipyretics
Diagnosis
·
Evidence of hypoglycaemia, metabolic
acidosis.
·
Evidence of hepatocellular injury:
o Elevated aspartate aminotransferase
(AST), alanine aminotransferase (ALT), bilirubin, and prothrombin time. ALT and
AST may rise within 24 hours after ingestion, and peak within 48 to 72 hours.
Levels over 10,000 units/L are common.
o Hypophosphataemia is often present.
Hyper phosphataemia (greater than 1.2 mmol/L), occurring 48 to 96 hours after
the overdose, and in the presence of both renal and hepatic dysfunction, is a
poor prognostic indicator.
o Decreased serum interleukin-6 (IL-6)
or C-reactive protein (a surrogate for IL-6) levels following acute paracetamol
overdose have been found to be statisti-cally associated with hepatic injury
and may serve as prognostic factors for predicting impending hepatic injury.
o Fatal cases of paracetamol overdose
usually have a bilirubin level greater than 4 mg/100 ml and a prothrombin time
greater than twice the control or a prothrombin time ratio of 2.2 or greater on
the third to the fifth day.
o Serum prealbumin concentrations
decrease significantly after 36 hours and continue to decrease during liver
failure, providing a true index of liver function.
·
Evidence of renal damage: Proteinuria, phosphaturia.
·
Evidence of myocardial damage: ECG changes indicativeof
arrrhythmias.
·
Serum paracetamol level: The lowest acute dose
ofparacetamol capable of toxicity is generally regarded as 7.5 grams in an
adult and 150 mg/kg in a child, though it is more likely that the actual
figures may be 15 grams and 250 mg/kg respectively.
·
Interpretation of serum paracetamol (acetami-nophen) levels
is usually done on the basis of the Rumack-Matthew nomogram (Fig 29.2). The orig-inal nomogram was
based on the fact that patients in whom the AST or ALT values would rise (more
than 1000 IU/L) could be separated from those in whom this would not happen on
the basis of initial paracetamol level (PL). The nomogram line was constructed
on a plot of PL versus time since inges-tion. The line chosen started at a PL
of 200 mcg/ml, 4 hours post-ingestion; declined with a 4-hour half-life through
50 mcg/ml, 12 hours post-ingestion; and ended at 6.25 mcg/ml, 24 hours
post-ingestion. The line is based solely on ALT elevation rather than hepatic
failure and is very sensitive though not very specific.
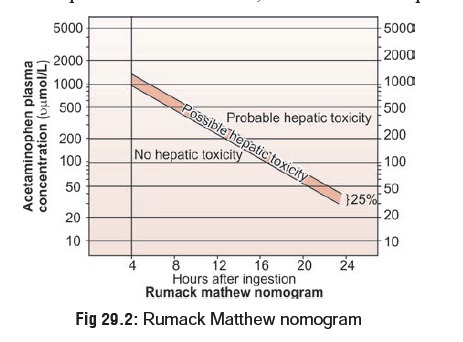
·
Without antidotal therapy (vide infra), 60% of the patients with initial PL above this line
would develop ALT values above 1000IU/L.
–– While the original line is still
widely in use, the line used in USA has been lowered by 25% in order to enhance
sensitivity. By using this modified nomo-gram it is said that nomogram failures
in the USA are virtually non-existent.
––
The purpose of the Rumack-Matthew nomogram is to predict the risk of
hepatic injury at the outset and begin antidotal therapy well in time. Levels
obtained before 4 hours or after 24 hours cannot be interpreted, nor can levels
obtained after chronic overdose. Though the risk cannot be predicted earlier
than 4 hours post-ingestion, this does not matter at all since there seems to
be no added effi-cacy in antidotal therapy when begun earlier than 4 hours
after the overdose.
–– In those cases where the time of
ingestion is unknown and cannot be determined, it is advis-able to determine
both PL and AST. If the AST is elevated regardless of PL, begin antidotal
therapy. If the PL is less than 10 mcg/ml and AST level is normal, the antidote
(N-acetylcysteine) can be withheld.
–– If it is not possible for any reason to estimate the paracetamol level at all, then the risk of hepatic injury should be predicted on the basis of the history. Do not delay antidotal therapy for lack of a paracetamol level.
Administer loading dose, then discontinue if level comes back below the
nomo-gram treatment line.
![]()
–– Conflicting reports are found in
the literature regarding whether or not a lower treatment line on the
Rumack-Matthew nomogram should be used for treating acute paracetamol overdoses
in chronic alcoholics. On the one hand, a review of the literature has shown in
animal studies that a lower dose of paracetamol is required to produce
hepatotoxicity following chronic alcohol use due to induction of CYP enzymes
and glutathione depletion. It is suggested that the animal results may apply to
human cases, and some authors suggest a conservative guess of halving the dose/
concentration for treatment. On the other hand, due to species differences in
CYP expression, activity and induction, results cannot always be extrapolated
from animals to human cases. Also, a literature review does not conclusively
substantiate that exposure to chronic excessive amounts of alcohol will
predispose paracetamol overdose patients to hepatotoxicity.
–– After 24 hours postingestion, the
presence of paracetamol in the plasma may be documented, but interpretation of
these levels is difficult. Because of increasing evidence of the beneficial
effect of N-acetylcysteine instituted more than 24 hours after overdose, its
use is recommended in patients presenting 24 hours or more post-ingestion who
have measurable paracetamol levels or biochemical evidence of hepatic injury.
·
One study compared a qualitative urine paracetamol screen
(thin-layer chromatography) to a qualitative serum paracetamol screen in several
patients following intentional ingestions. It was found that a negative urine
paracetamol was highly predictive of negative serum paracetamol levels. This
suggests that a negative urine screen may obviate the need for 4-hour
quantitative serum levels.
·
Concomitantly ingested drugs which change the rate of
gastric emptying (codeine, other opiates, antimuscarinic drugs,
antihistamines), may delay absorption of paracet-amol. Additional levels may be
needed to determine the peak and the need for antidote.
·
Assessment of prognosis—Poor prognosis is characteristic of
o pH < 7.30 in spite of fluid and
haemodynamic resuscita-tion.
o PT > 100 seconds, creatinine > 3.3 mg/100 ml, and grade III or IV
encephalopathy.
o Abnormal PT which continues to rise
on the 4th day. In late presenters following paracetamol overdose, the best
prognostic marker in established hepatotox-icity is the prothrombin time.
Extended courses of ![]() N-acetylcysteine may be given until
the prothrombin time improves.
N-acetylcysteine may be given until
the prothrombin time improves.
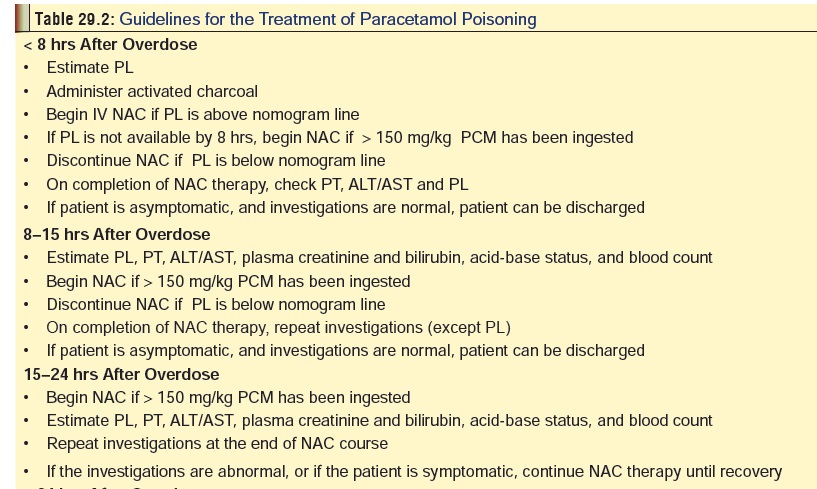
Treatment (Table 29.2)
Children who have an unobtainable
history or in whom a large amount of paracetamol is suspected to have been
ingested (>200 mg/kg) should be referred to a health care facility for a
4-hour paracetamol serum level determination, and consideration for administration
of activated charcoal.

·
Stomach wash: useful only in cases of very early
presen-tation (<1 hour), or in concomitant ingestion of other drugs which
delay GI absorption.
·
Activated charcoal can adsorb paracetamol, but it
canalso adsorb the antidote (N-acetylcysteine) and hence must be administered
earlier to 4 hours post-ingestion. It is most effective if given within one
hour of ingestion of a liquid formulation or a tablet formulation.
·
Anti-emetic, if the patient is vomiting
repeatedly.
·
Supportive measures:
o 10 to 20% dextrose for
hypoglycaemia.
o Vitamin K1 if PT is
elevated.
o Fresh-frozen plasma if there is
overt bleeding.
o Mannitol (0.5 gm/kg given over 10
minutes) for cerebral oedema.
o Broad spectrum antibiotics IV (ceftazidime or fluoxa-cillin) if necessary.
o H2 antagonists to prevent
upper GI haemorrhage.
o Do not give sedatives, benzodiazepines, or NSAIDs.
Antidotal therapy:
·
Methionine—This is an oral antidote
that is popular in the UK and some other countries, but is presently not
available in India. It is a glutathione precursor and protects against
paracetamol-induced hepatic and renal damage, provided it is administered
within 8 to 10 hours of ingestion. Dose: 2.5 grams, 4 doses, at 4-hour intervals.
·
N-acetylcysteine (NAC)—It is a
derivative of
·
L-cysteine, a naturally occuring
amino acid. NAC is the antidote of choice for paracetamol poisoning in the USA,
and is gaining in popularity elsewhere including India. It gives maximum
protection against hepatotoxicity when administered within 10 hours of
paracetamol overdose, but can be given with (lesser) benefit upto 36 hours.
––
Indications:
--
NAC is indicated in paracetamol overdose if PL estimated between 4 and
12 hours post-ingestion lies above the nomogram line (Fig 29.2).
-- Paracetamol ingested is more than
100 mg/kg. -- Likelihood exists of paracetamol-induced hepatic failure.
––
Dose—
-- Oral: 5% solution given as
loading dose of 140 mg/kg. This is followed by 17 more doses at 70 mg/kg, 4th
hourly, total making upto 1330 mg/kg over 72 hours. The calcu-lated dose can be
administered mixed with water, or flavoured or carbonated drink. Fora child: Initial - 140 mg/kg for one
dose,followed by 70 mg/kg/dose every 4 hours for 68 hours, beginning within 10
hours of the ingestion. A shorter duration of oral NAC has been recommended by
some investigators for acute paracetamol overdoses presenting within 24 hours
of ingestion: loading dose of 140 mg/kg followed by 70 mg/kg every 4 hours
until the serum paracetamol level is no longer detectable and aminotransferase
levels are normal, instead of the standard 72-hour treatment protocol. These
investigators found this method safe and effective in patients not
demonstrating hepatotoxicity within 36 hours of an acute overdose.
––
Intravenous—
-- 20 hour regimen (Prescott protocol) → 150 mg/kg made up in 200 ml of 5%
dextrose is given IV over 15 minutes, followed by 50 mg/ kg in 500 ml of 5%
dextrose over 4 hours, and 100 mg/kg in 1 litre of 5% dextrose over 16 hours.
The total dose works out to 300 mg/ kg given over 20 hours. For a child: Standard intravenous dosing
can cause hyponatraemia and seizures secondary to large amounts of free water.
To avoid this complication, NAC should be diluted to a final concentration of
40 mg/ml. 150 mg/kg infused over 15 minutes (infuse 3.75 ml/kg over 15
minutes), followed by 50 mg/kg infused over 4 hours (infuse 1.25 ml/kg over 4
hours, i.e., 0.31 ml/kg/hr), and 100 mg/kg infused over 16 hours (infuse 2.5
ml/kg over 16 hours, i.e. 0.16 ml/kg/hr).
-- 48 hour regimen → useful in delayed admis-sions and
massive ingestion. A loading dose of 140 mg IV is given over 1 hour, followed 4
hours later by the first of 12 maintenance doses of 70 mg/kg, each administered
over 1 hour. The total dose of NAC works out to 980 mg/kg in just over 48
hours.
––
Adverse effects:
-- Oral—drinking NAC through a straw
mini-mises its unpleasant odour. The main problem with oral NAC is induction of
vomiting. Metoclopramide or ondansetron may have to be administered.
-- Intravenous—anaphylactoid
reaction. If it occurs, it should be managed in the usual way with
antihistamines, epinephrine, etc.
--
Isolated effects include pruritus, angi-oedema, nausea and vomiting,
bronchospasm, tachycardia, hypotension, and hypertension. Facial or chest
flushing is common, beginning 15 to 75 minutes after initiation of infusion,
and is associated with peak NAC plasma concentrations of 100 to 600 mcg/L.![]()
--A decrease in the prothrombin
index (which corresponds to an increase in prothrombin time or INR) has been
reported following administration of IV NAC for treatment of patients with
paracetamol poisoning who did not exhibit signs of hepatocellular injury. The
time of the decrease appeared to be associated with the start of the NAC
infusion instead of with the ingestion of paracetamol. Because prothrombin time
is measured as a prog-nostic indicator in patients with paracetamol poisoning,
the concern is that the decrease in prothrombin index may be misinterpreted as
a sign of liver failure. It is suggested that patient management decisions
should not be based solely on the measurement of this value.
o Liver transplantation: When fulminant liver
failuredevelops after a massive paracetamol overdose, virtually the only
treatment modality available is liver transplantation.
a.
Indications— –– pH < 7.3.
–– PT > 100 seconds, and serum creatinine > 3.4 mg/100 ml in patients with
grade III or IV encephalopathy.
–– Schiodt et al (1999) developed a model, based on a prospective
and validated study, to predict hepatic encephalopathy (HE) in paracetamol
overdose, and to identify high-risk patients for early transfer to a liver
intensive care unit/trans-plantation facility. The most accurate model for HE
included: log10 (hours from overdose to antidote treatment), log10 (plasma
coagulation factors on admission), and platelet count × hours from overdose
(chi-square = 41.2, P<0.00001). HE was not seen in patients treated within
18 hours after overdose. A good predictor of later hepatic encephalopathy also
includes a total Gc-globulin level less than 120 mg/L on day 2 following
paracetamol overdose. This value was based on a prospective longitudinal study
including 84 patients with acute paracetamol overdose.
––
The O’Grady criteria is a
multivariate prog-nostic scoring system for predicting the need for listing a
patient for liver transplantation.
The criteria include: arterial blood
pH < 7.3 or H+ >50 mmol/L; or, PTR >100 seconds and serum creatinine
>300 mcmol/L in patients with Grade III or IV encephalopathy. A modifiedO’Grady criteria states that if
serum lactate is>3 at 4 hours, or >3.5 at 12 hours (after initial fluid
resuscitation), the positive predictive value ![]() of the O’Grady criteria is
increased. A high APACHE (Acute Physiologic and Chronic Health Evaluation) II
or III score may also predict the need for liver transplantation.
of the O’Grady criteria is
increased. A high APACHE (Acute Physiologic and Chronic Health Evaluation) II
or III score may also predict the need for liver transplantation.
––
The use of arterial lactate concentration may allow for earlier
identification of patients at high-risk of fatal paracetamol induced liver
failure and likely to benefit from listing early for liver transplantation.
Some investigators have found that an early arterial lactate 4 hours after
transfer (median of 43 hours after ingestion) above 3.5 mmol/L correlated with
an increased risk of fatal outcome. An arterial lactate concentration 12 hours
after transfer and after adequate fluid resuscitation (guided by invasive
haemodynamic monitoring) above 3.0 mmol/L also correlated with an increased
risk of fatality. All patients had ICP monitoring as appropriate, noradrenaline
was used as the primary vasopressor, NAC was infused at 150 mg/kg for 24 hours,
and continuous venovenous haemofiltration with lactate-free fluid was used for
renal replacement.
–– Bernal et al have proposed criteria for liver transplantation in
paracetamol-induced acute liver failure as follows:
--strongly consider listing for
transplantation if arterial lactate concentration >3.5 mmol/L after early
fluid resuscitation
--list for transplantation if
arterial pH <7.3 mmol/L, or arterial lactate concentration >3.0 mmol/L
after adequate fluid resuscitation.
--or concurrently serum creatinine
>300 mcmol/L, INR >6.5 and there is encepha-lopathy of grade 3 or
greater.
·
Forced diuresis, haemodialysis, and charcoal haemo-perfusion are of little value in preventing
paracetamolinduced hepatotoxicity.
·
Albumin dialysis: A molecular adsorbent
recirculatingsystem (MARS), which is a modified dialysis method using an
albumin-containing dialysate that is recirculated and perfused online through
charcoal and anion-exchange columns, has been used following a massive
paracetamol overdose with hepatic encephalopathy (grade II), severe acidosis,
INR of 7, and hepatorenal syndrome. Albumin dialysis allowed time for hepatic
regeneration during conventional supportive care in this case. A course of 5
consecutive 8-hour treatments was performed.
·
Continuous haemofiltration: This may be preferable
tointermittent haemodialysis in patients with paracetamol-induced hepatic and
renal failure. Use of intermittent haemodialysis is associated with increases
in intracra-nial pressure in these patients due to both cytotoxic and vasogenic
cerebral oedema.
·
Extracorporeal sorbent-based devices: Paracetamol-induced hepatitis or
hepatic failure have been treated at 16 to 68 hours after an overdose for 4 to
6 hours with the Liver Dialysis System (a single-access haemodiab-sorption
system for treatment of serious drug overdose, and for treatment of hepatic
encephalopathy). During this treatment, paracetamol levels dropped an average
of 73%. If paracetamol levels were still measurable in plasma, treatment was
repeated 24 or 48 hours later. In this group, liver enzymes normalised 24 hours
after the last treatment and no patient required a liver transplant. No adverse
effects due to this treatment were noted.
Related Topics