Chapter: Medical Physiology: Regulation of Extracellular Fluid Osmolarity and Sodium Concentration
Role of Thirst in Controlling Extracellular Fluid Osmolarity and Sodium Concentration
Role of Thirst in Controlling Extracellular Fluid Osmolarity and Sodium Concentration
The kidneys minimize fluid loss during water deficits through the osmoreceptor-ADH feedback system. Adequate fluid intake, however, is necessary to coun-terbalance whatever fluid loss does occur through sweating and breathing and through the gastrointesti-nal tract. Fluid intake is regulated by the thirst mech-anism, which, together with the osmoreceptor-ADH mechanism, maintains precise control of extracellular fluid osmolarity and sodium concentration.
Many of the same factors that stimulate ADH secre-tion also increase thirst, which is defined as the con-scious desire for water.
Central Nervous System Centers for Thirst
Referring again to Figure 28–9, the same area along the anteroventral wall of the third ventricle that pro-motes ADH release also stimulates thirst. Located anterolaterally in the preoptic nucleus is another small area that, when stimulated electrically, causes immedi-ate drinking that continues as long as the stimulation lasts. All these areas together are called the thirstcenter.
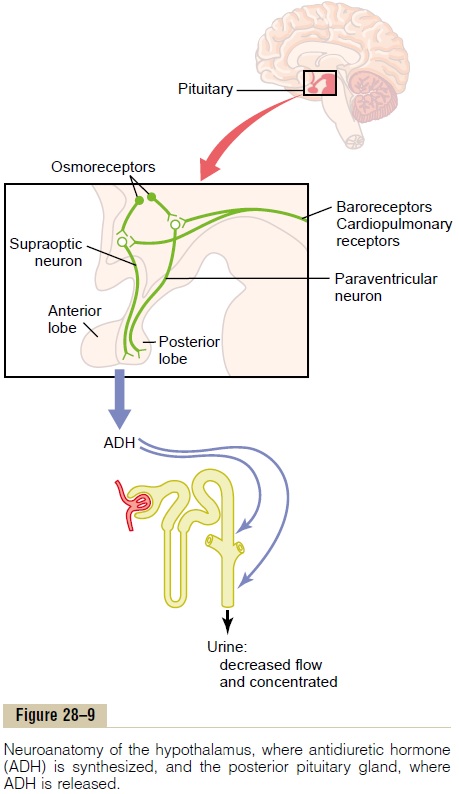
The neurons of the thirst center respond to injec-tions of hypertonic salt solutions by stimulating drink-ing behavior. These cells almost certainly function as osmoreceptors to activate the thirst mechanism, in the same way that the osmoreceptors stimulate ADH release.
Increased osmolarity of the cerebrospinal fluid in the third ventricle has essentially the same effect to promote drinking. It is likely that the organum vascu-losum of the lamina terminalis, which lies immediatelybeneath the ventricular surface at the inferior end of the AV3V region, is intimately involved in mediating this response.
Stimuli for Thirst
Table 28–3 summarizes some of the known stimuli for thirst. One of the most important is increased extra-cellular fluid osmolarity, which causes intracellular dehydration in the thirst centers, thereby stimulatingthe sensation of thirst. The value of this response is obvious: it helps to dilute extracellular fluids and returns osmolarity toward normal.
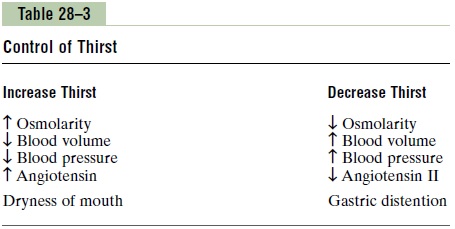
Decreases in extracellular fluid volume and arterial pressure also stimulate thirst by a pathway that is inde-pendent of the one stimulated by increased plasma osmolarity. Thus, blood volume loss by hemorrhage stimulates thirst even though there might be no change in plasma osmolarity. This probably occurs because of neutral input from cardiopulmonary and systemic arterial baroreceptors in the circulation.
A third important stimulus for thirst is angiotensin II.Studies in animals have shown that angiotensin II acts on the subfornical organ and on the organum vascu-losum of the lamina terminalis. These regions are outside the blood-brain barrier, and peptides such as angiotensin II diffuse into the tissues. Because angiotensin II is also stimulated by factors associated with hypovolemia and low blood pressure, its effect on thirst helps to restore blood volume and blood pres-sure toward normal, along with the other actions of angiotensin II on the kidneys to decrease fluid excretion.
Dryness of the mouth and mucous membranes of the esophagus can elicit the sensation of thirst. As a result,a thirsty person may receive relief from thirst almost immediately after drinking water, even though the water has not been absorbed from the gastrointestinal tract and has not yet had an effect on extracellular fluid osmolarity.
Gastrointestinal and pharyngeal stimuli influence thirst. For example, in animals that have an esophagealopening to the exterior so that water is never absorbed into the blood, partial relief of thirst occurs after drinking, although the relief is only temporary. Also, gastrointestinal distention may partially alleviate thirst; for instance, simple inflation of a balloon in the stomach can relieve thirst. However, relief of thirst sensations through gastrointestinal or pharyngeal mechanisms is short-lived; the desire to drink is com-pletely satisfied only when plasma osmolarity and/or blood volume returns to normal.
The ability of animals and humans to “meter” fluid intake is important because it prevents overhydration. After a person drinks water, 30 to 60 minutes may be required for the water to be reabsorbed and dis-tributed throughout the body. If the thirst sensation were not temporarily relieved after drinking water, the person would continue to drink more and more, even-tually leading to overhydration and excess dilution of the body fluids. Experimental studies have repeatedly shown that animals drink almost exactly the amount necessary to return plasma osmolarity and volume to normal.
Threshold for Osmolar Stimulus of Drinking
The kidneys must continually excrete at least some fluid, even in a dehydrated person, to rid the body of excess solutes that are ingested or produced by metab-olism. Water is also lost by evaporation from the lungs and the gastrointestinal tract and by evaporation and sweating from the skin. Therefore, there is always a tendency for dehydration, with resultant increased extracellular fluid sodium concentration and osmolar-ity. When the sodium concentration increases only about 2 mEq/L above normal, the thirst mechanism is activated, causing a desire to drink water. This is called the threshold for drinking. Thus, even small increases in plasma osmolarity are normally followed by water intake, which restores extracellular fluid osmolarity and volume toward normal. In this way, the extracel-lular fluid osmolarity and sodium concentration are precisely controlled.
Integrated Responses of Osmoreceptor-ADH and Thirst Mechanisms in Controlling Extracellular Fluid Osmolarity and Sodium Concentration
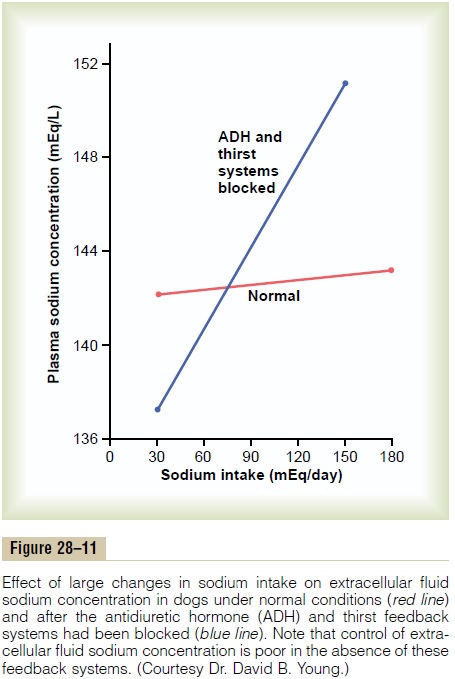
In a healthy person, the osmoreceptor-ADH and thirst mechanisms work in parallel to precisely regulate extracellular fluid osmolarity and sodium concentra-tion, despite the constant challenges of dehydration. Even with additional challenges, such as high salt intake, these feedback systems are able to keep plasma osmolarity reasonably constant. Figure 28–11 shows that an increase in sodium intake to as high as six times normal has only a small effect on plasma sodium con-centration as long as the ADH and thirst mechanisms are both functioning normally.
When either the ADH or the thirst mechanism fails, the other ordinarily can still control extracellular osmolarity and sodium concentration with reasonable effectiveness, as long as there is enough fluid intake to balance the daily obligatory urine volume and water losses caused by respiration, sweating, or gastrointestinal losses. However, if both the ADH and thirst mechanisms fail simultaneously, plasma sodium concentration and osmolarity are very poorly controlled; thus, when sodium intake is increased after blocking the total ADH-thirst system, relatively large changes in plasma sodium concentration do occur. In the absence of the ADH-thirst mechanisms, no other feedback mechanism is capable of ad-equately regulating plasma sodium concentration and osmolarity.
Role of Angiotensin II and Aldosterone in Controlling Extracellular Fluid Osmolarity and Sodium Concentration
As discussed, both angiotensin II and aldosterone play an important role in regulating sodium reabsorption by the renal tubules. When sodium intake is low, increased levels of these hormones stimulate sodium reabsorption by the kidneys and, therefore, prevent large sodium losses, even though sodium intake may be reduced to as low as 10 per cent of normal. Con-versely, with high sodium intake, decreased formation of these hormones permits the kidneys to excrete large amounts of sodium.
Because of the importance of angiotensin II and aldosterone in regulating sodium excretion by the kidneys, one might mistakenly infer that they also play an important role in regulating extracellular fluid sodium concentration. Although these hormones increase the amount of sodium in the extracellular fluid, they also increase the extracellular fluid volume by increasing reabsorption of water along with the sodium. Therefore, angiotensin II and aldosterone have littleeffect on sodium concentration, except under extreme conditions.
This relative unimportance of aldosterone in regulat-ing extracellular fluid sodium concentration is shown by the experiment of Figure 28–12. This figure shows the effect on plasma sodium concentration of changing sodium intake more than sixfold under two conditions:
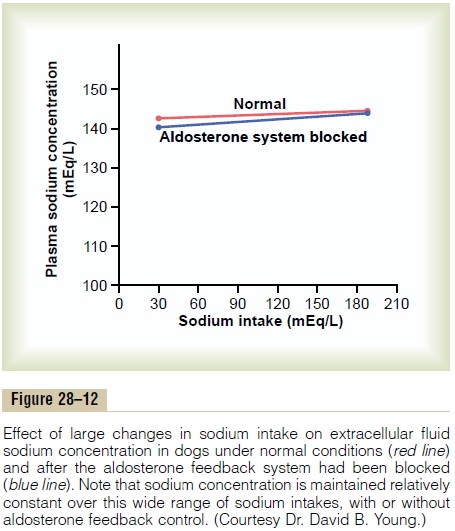
(1) under normal conditions and (2) after the aldos-terone feedback system has been blocked by removing the adrenal glands and infusing the animals with aldos-terone at a constant rate so that plasma levels could not change upward or downward. Note that when sodium intake was increased sixfold, plasma concentration changed only about 1 to 2 per cent in either case. This indicates that even without a functional aldosterone feedback system, plasma sodium concentration can be well regulated. The same type of experiment has been conducted after blocking angiotensin II formation, with the same result.
There are two primary reasons why changes in angiotensin II and aldosterone do not have a major effect on plasma sodium concentration. First, as dis-cussed earlier, angiotensin II and aldosterone increase both sodium and water reabsorption by the renal tubules, leading to increases in extracellular fluid volume and sodium quantity but little change in sodium concentrations. Second, as long as the ADH-thirst mech-anism is functional, any tendency toward increased plasma sodium concentration is compensated for by increased water intake or increased plasma ADH secre-tion, which tends to dilute the extracellular fluid back toward normal. The ADH-thirst system far overshad-ows the angiotensin II and aldosterone systems for reg-ulating sodium concentration under normal conditions. Even in patients with primary aldosteronism, who have extremely high levels of aldosterone, the plasma sodium concentration usually increases only about 3 to 5 mEq/L above normal.
Under extreme conditions, caused by complete loss of aldosterone secretion because of adrenalectomy or in patients with Addison’s disease (severely impaired secretion or total lack of aldosterone), there is tremen-dous loss of sodium by the kidneys, which can lead to reductions in plasma sodium concentration. One of the reasons for this is that large losses of sodium eventually cause severe volume depletion and decreased blood pressure, which can activate the thirst mechanism through the cardiovascular reflexes. This leads to a further dilution of the plasma sodium concentration, even though the increased water intake helps to mini-mize the decrease in body fluid volumes under these conditions.
Thus, there are extreme situations in which plasma sodium concentration may change significantly, even with a functional ADH-thirst mechanism. Even so, the ADH-thirst mechanism is by far the most powerful feedback system in the body for controlling extracellu-lar fluid osmolarity and sodium concentration.
Related Topics