Chapter: Medical Physiology: Regulation of Extracellular Fluid Osmolarity and Sodium Concentration
Kidneys Conserve Water by Excreting a Concentrated Urine
The Kidneys Conserve Water by Excreting a Concentrated Urine
The ability of the kidney to form a urine that is more concentrated than plasma is essential for survival of mammals that live on land, including humans. Water is continuously lost from the body through various routes, including the lungs by evaporation into the expired air, the gastrointestinal tract by way of the feces, the skin through evaporation and perspiration, and the kidneys through the excretion of urine. Fluid intake is required to match this loss, but the ability of the kidney to form a small volume of concentrated urine minimizes the intake of fluid required to main-tain homeostasis, a function that is especially impor-tant when water is in short supply.
When there is a water deficit in the body, the kidney forms a concentrated urine by continuing to excrete solutes while increasing water reabsorption and decreasing the volume of urine formed. The human kidney can produce a maximal urine concentration of 1200 to 1400 mOsm/L, four to five times the osmolar-ity of plasma. Some desert animals, such as the Aus-tralian hopping mouse, can concentrate urine to as high as 10,000 mOsm/L. This allows the mouse to survive in the desert without drinking water; sufficient water can be obtained through the food ingested and water produced in the body by metabolism of the food. Animals adapted to aquatic environments, such as the beaver, have minimal urine concentrating ability; they can concentrate the urine to only about 500 mOsm/L.
Obligatory Urine Volume
The maximal concentrating ability of the kidney dic-tates how much urine volume must be excreted each day to rid the body of waste products of metabolism and ions that are ingested. A normal 70-kilogram human must excrete about 600 milliosmoles of solute each day. If maximal urine concentrating ability is 1200 mOsm/L, the minimal volume of urine that must be excreted, called the obligatory urine volume, can be calculated as
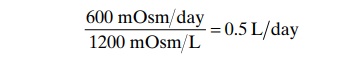
This minimal loss of volume in the urine contributes to dehydration, along with water loss from the skin, respi-ratory tract, and gastrointestinal tract, when water is not available to drink.
The limited ability of the human kidney to con-centrate the urine to a maximal concentration of 1200 mOsm/L explains why severe dehydration occurs if one attempts to drink seawater. Sodium chloride concentration in the oceans averages about 3.0 to 3.5 per cent, with an osmolarity between about 1000 and 1200 mOsm/L. Drinking 1 liter of seawater with a con-centration of 1200 mOsm/L would provide a total sodium chloride intake of 1200 milliosmoles. If maximal urine concentrating ability is 1200 mOsm/L, the amount of urine volume needed to excrete 1200 milliosmoles would be 1200 milliosmoles divided by 1200 mOsm/L, or 1.0 liter. Why then does drinking seawater cause dehydration? The answer is that the kidney must also excrete other solutes, especially urea, which contribute about 600 mOsm/L when the urine is maximally con-centrated. Therefore, the maximum concentration of sodium chloride that can be excreted by the kidneys is about 600 mOsm/L. Thus, for every liter of seawater drunk, 2 liters of urine volume would be required to rid the body of 1200 milliosmoles of sodium chloride ingested in addition to other solutes such as urea. This would result in a net fluid loss of 1 liter for every liter of seawater drunk, explaining the rapid dehydration that occurs in shipwreck victims who drink seawater. However, a shipwreck victim’s pet Australian hopping mouse could drink with impunity all the seawater it wanted.
Requirements for Excreting a Concentrated Urine—High ADH Levels and Hyperosmotic Renal Medulla
The basic requirements for forming a concentrated urine are (1) a high level of ADH, which increases the permeability of the distal tubules and collecting ducts to water, thereby allowing these tubular segments to avidly reabsorb water, and (2) a high osmolarity of therenal medullary interstitial fluid, which provides theosmotic gradient necessary for water reabsorption to occur in the presence of high levels of ADH.
The renal medullary interstitium surrounding the collecting ducts normally is very hyperosmotic, so that when ADH levels are high, water moves through the tubular membrane by osmosis into the renal intersti-tium; from there it is carried away by the vasa recta back into the blood. Thus, the urine concentrating ability is limited by the level of ADH and by the degree of hyperosmolarity of the renal medulla. We discuss the factors that control ADH secretion later, but for now, what is the process by which renal medullary interstitial fluid becomes hyperosmotic? This process involves the operation of thecountercur-rent mechanism.
The countercurrent mechanism depends on the special anatomical arrangement of the loops of Henle and the vasa recta, the specialized peritubular capillar-ies of the renal medulla. In the human, about 25 percent of the nephrons are juxtamedullary nephrons, with loops of Henle and vasa recta that go deeply into the medulla before returning to the cortex. Some of the loops of Henle dip all the way to the tips of the renal papillae that project from the medulla into the renal pelvis. Paralleling the long loops of Henle are the vasa recta, which also loop down into the medulla before returning to the renal cortex. And finally, the collecting ducts, which carry urine through the hyper-osmotic renal medulla before it is excreted, also play a critical role in the countercurrent mechanism.
Countercurrent Mechanism Produces a Hyperosmotic Renal Medullary Interstitium
The osmolarity of interstitial fluid in almost all parts of the body is about 300 mOsm/L, which is similar to the plasma osmolarity. (the corrected osmolar activity, which accounts for intermolecular attraction and repulsion, is about 282 mOsm/L.) The osmolarity of the interstitial fluid in the medulla of the kidney is much higher, increas-ing progressively to about 1200 to 1400 mOsm/L in the pelvic tip of the medulla. This means that the renal medullary interstitium has accumulated solutes in great excess of water. Once the high solute concentra-tion in the medulla is achieved, it is maintained by a balanced inflow and outflow of solutes and water in the medulla.
The major factors that contribute to the buildup of solute concentration into the renal medulla are as follows:
1. Active transport of sodium ions and co-transport of potassium, chloride, and other ions out of the thick portion of the ascending limb of the loop of Henle into the medullary interstitium.
2. Active transport of ions from the collecting ducts into the medullary interstitium
3. Facilitated diffusion of large amounts of urea from the inner medullary collecting ducts into the medullary interstitium
4. Diffusion of only small amounts of water from the medullary tubules into the medullary interstitium, far less than the reabsorption of solutes into the medullary interstitium
Special Characteristics of Loop of Henle That Cause Solutes to Be Trapped in the Renal Medulla. The transport character-istics of the loops of Henle are summarized in Table 28–1, along with the characteristics of the proximal tubules, distal tubules, cortical collecting tubules, and inner medullary collecting ducts.
The most important cause of the high medullary osmolarity is active transport of sodium and co-transport of potassium, chloride, and other ions from the thick ascending loop of Henle into the interstitium. This pump is capable of establishing about a 200-milliosmole concentration gradient between the tubular lumen and the interstitial fluid. Because the thick ascending limb is virtually impermeable to water, the solutes pumped out are not followed by osmotic flow of water into the interstitium. Thus, the active transport of sodium and other ions out of the thick ascending loop adds solutes in excess of water to the renal medullary interstitium. There is some passive reabsorption of sodium chloride from the thin ascend-ing limb of Henle’s loop, which is also impermeable to water, adding further to the high solute concentration of the renal medullary interstitium.
The descending limb of Henle’s loop, in contrast to the ascending limb, is very permeable to water, and the tubular fluid osmolarity quickly becomes equal to the renal medullary osmolarity. Therefore, water diffuses out of the descending limb of Henle’s loop into the interstitium, and the tubular fluid osmolarity gradually rises as it flows toward the tip of the loop of Henle.
Steps Involved in Causing Hyperosmotic Renal Medullary Inter-stitium. With these characteristics of the loop of Henlein mind, let us now discuss how the renal medulla becomes hyperosmotic. First, assume that the loop of Henle is filled with fluid with a concentration of 300 mOsm/L, the same as that leaving the proximal tubule (Figure 28–3, step 1). Next, the active pump of the thick ascending limb on the loop of Henle is turned on, reducing the concentration inside the tubule and
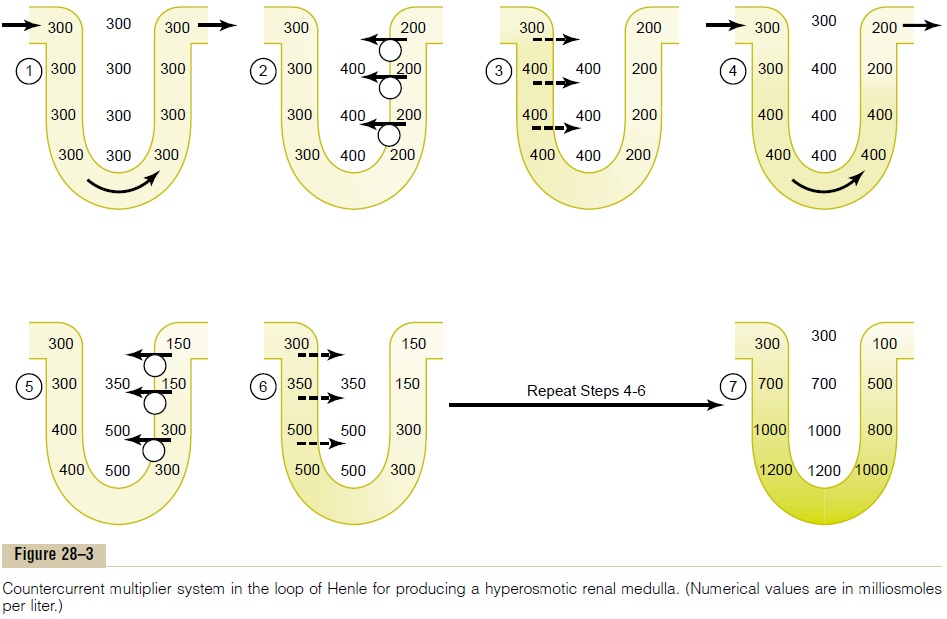
raising the interstitial concentration; this pump estab-lishes a 200-mOsm/L concentration gradient between the tubular fluid and the interstitial fluid (step 2). The limit to the gradient is about 200 mOsm/L because paracellular diffusion of ions back into the tubule eventually counterbalances transport of ions out of the lumen when the 200-mOsm/L concentration gradient is achieved.
Step 3 is that the tubular fluid in the descending limbof the loop of Henle and the interstitialfluid quicklyreach osmotic equilibrium because of osmosis of water out of the descending limb. The interstitial osmolarity is maintained at 400 mOsm/L because of continued transport of ions out of the thick ascending loop of Henle. Thus, by itself, the active transport of sodium chloride out of the thick ascending limb is capable of establishing only a 200-mOsm/L concentration gradi-ent, much less than that achieved by the countercur-rent system.
Step 4 is additional flow of fluid into the loop of Henle from the proximal tubule, which causes the hyperosmotic fluid previously formed in the descend-ing limb to flow into the ascending limb. Once this fluid is in the ascending limb, additional ions are pumped into the interstitium, with water remaining behind, until a 200-mOsm/L osmotic gradient is established, with the interstitial fluid osmolarity rising to 500 mOsm/L (step 5). Then, once again, the fluid in the descending limb reaches equilibrium with the hyper-osmotic medullary interstitial fluid (step 6), and as the hyperosmotic tubular fluid from the descending limb of the loop of Henle flows into the ascending limb, still more solute is continuously pumped out of the tubules and deposited into the medullary interstitium.
These steps are repeated over and over, with the net effect of adding more and more solute to the medulla in excess of water; with sufficient time, this processgradually traps solutes in the medulla and multiplies the concentration gradient established by the active pumping of ions out of the thick ascending loop of Henle, eventually raising the interstitial fluid osmolar-ity to 1200 to 1400 mOsm/L as shown in step 7.
Thus, the repetitive reabsorption of sodium chloride by the thick ascending loop of Henle and continued inflow of new sodium chloride from the proximal tubule into the loop of Henle is called the countercur-rent multiplier. The sodium chloride reabsorbed fromthe ascending loop of Henle keeps adding to the newly arrived sodium chloride, thus “multiplying” its con-centration in the medullary interstitium.
Role of Distal Tubule and Collecting Ducts in Excreting a Concentrated Urine
When the tubular fluid leaves the loop of Henle and flows into the distal convoluted tubule in the renal cortex, the fluid is dilute, with an osmolarity of only about 100 mOsm/L (Figure 28–4). The early distal
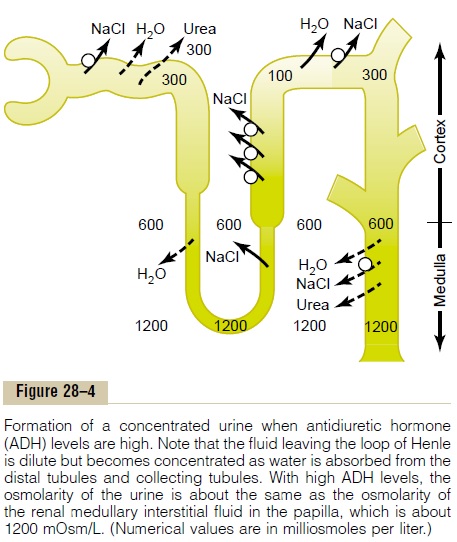
tubule further dilutes the tubular fluid because this segment, like the ascending loop of Henle, actively transports sodium chloride out of the tubule but is rel-atively impermeable to water.
As fluid flows into the cortical collecting tubule, the amount of water reabsorbed is critically dependent on the plasma concentration of ADH. In the absence of ADH, this segment is almost impermeable to water and fails to reabsorb water but continues to reabsorb solutes and further dilutes the urine. When there is a high concentration of ADH, the cortical collecting tubule becomes highly permeable to water, so that large amounts of water are now reabsorbed from the tubule into the cortex interstitium, where it is swept away by the rapidly flowing peritubular capillaries.
The fact that these large amounts of water are reab-sorbed into the cortex, rather than into the renal medulla, helps to preserve the high medullary intersti-tial fluid osmolarity.
As the tubular fluid flows along the medullary col-lecting ducts, there is further water reabsorption from the tubular fluid into the interstitium, but the total amount of water is relatively small compared with that added to the cortex interstitium. The reabsorbed water is quickly carried away by the vasa recta into the venous blood. When high levels of ADH are present, the collecting ducts become permeable to water, so that the fluid at the end of the collecting ducts has essentially the same osmolarity as the interstitial fluid of the renal medulla—about 1200 mOsm/L (see Figure 28–3). Thus, by reabsorbing as much water as possible, the kidneys form a highly concentrated urine, excret-ing normal amounts of solutes in the urine while adding water back to the extracellular fluid and com-pensating for deficits of body water.
Urea Contributes to Hyperosmotic Renal Medullary Interstitium and to a Concentrated Urine
Thus far, we have considered only the contribution of sodium chloride to the hyperosmotic renal medullary interstitium. However, urea contributes about 40 to 50 per cent of the osmolarity (500-600 mOsm/L) of the renal medullary interstitium when the kidney is forming a maximally concentrated urine. Unlike sodium chloride, urea is passively reabsorbed from the tubule. When there is water deficit and blood concen-trations of ADH are high, large amounts of urea are passively reabsorbed from the inner medullary col-lecting ducts into the interstitium.
The mechanism for reabsorption of urea into the renal medulla is as follows: As water flows up the ascending loop of Henle and into the distal and corti-cal collecting tubules, little urea is reabsorbed because these segments are impermeable to urea (see Table 28–1). In the presence of high concentrations of ADH, water is reabsorbed rapidly from the cortical col-lecting tubule and the urea concentration increases rapidly because urea is not very permeant in this part of the tubule. Then, as the tubular fluid flows into the inner medullary collecting ducts, still more water reab-sorption takes place, causing an even higher concen-tration of urea in the fluid. This high concentration of urea in the tubular fluid of the inner medullary col-lecting duct causes urea to diffuse out of the tubule into the renal interstitium. This diffusion is greatly facilitated by specific urea transporters. One of these urea transporters, UT-AI, is activated by ADH, increasing transport of urea out of the inner medullary collecting duct even more when ADH levels are ele-vated. The simultaneous movement of water and urea out of the inner medullary collecting ducts maintains a high concentration of urea in the tubular fluid and, eventually, in the urine, even though urea is being reabsorbed.
The fundamental role of urea in contributing to urine concentrating ability is evidenced by the fact that people who ingest a high-protein diet, yielding large amounts of urea as a nitrogenous “waste” product, can concentrate their urine much better than people whose protein intake and urea production are low. Malnutrition is associated with a low urea concentra-tion in the medullary interstitium and considerable impairment of urine concentrating ability.
Recirculation of Urea from Collecting Duct to Loop of Henle Contributes to Hyperosmotic Renal Medulla. A personusually excretes about 20 to 50 per cent of the filtered load of urea. In general, the rate of urea excretion is determined mainly by two factors: (1) the concentra-tion of urea in the plasma and (2) the glomerular fil-tration rate (GFR). In patients with renal disease who have large reductions of GFR, the plasma urea con-centration increases markedly, returning the filtered urea load and urea excretion rate to the normal level (equal to the rate of urea production), despite the reduced GFR.
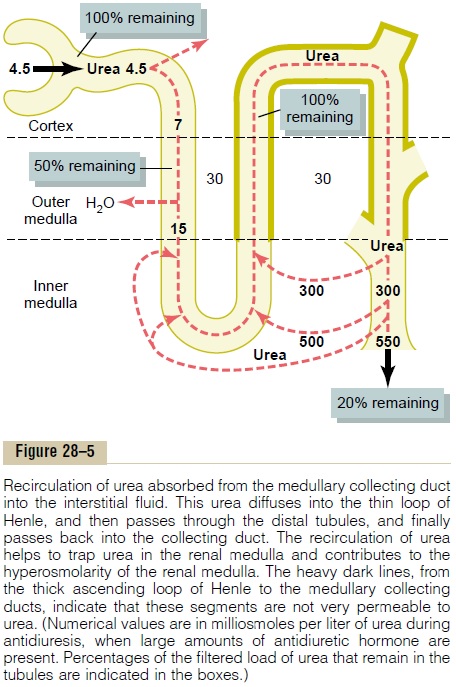
In the proximal tubule, 40 to 50 per cent of the fil-tered urea is reabsorbed, but even so, the tubular fluid urea concentration increases because urea is not nearly as permeant as water. The concentration of urea continues to rise as the tubular fluid flows into the thin segments of the loop of Henle, partly because of water reabsorption out of the descending loop of Henle but also because of some secretion of urea into the thin loop of Henle from the medullary interstitium (Figure 28–5).
The thick limb of the loop of Henle, the distal tubule, and the cortical collecting tubule are all rela-tively impermeable to urea, and very little urea reab-sorption occurs in these tubular segments. When the kidney is forming a concentrated urine and high levels of ADH are present, the reabsorption of water from the distal tubule and cortical collecting tubule further raises the tubular fluid concentration of urea. And as this urea flows into the inner medullary collecting duct, the high tubular fluid concentration of urea and specific urea transporters cause urea to diffuse into the medullary interstitium. A moderate share of the urea that moves into the medullary interstitium eventually diffuses into the thin loop of Henle, so that it passes upward through the ascending loop of Henle, the distal tubule, the cortical collecting tubule, and back down into the medullary collecting duct again. In this way, urea can recirculate through these terminal parts of the tubular system several times before it is excreted. Each time around the circuit contributes to a higher concentration of urea.
This urea recirculation provides an additional mech-anism for forming a hyperosmotic renal medulla. Because urea is one of the most abundant waste prod-ucts that must be excreted by the kidneys, this mech-anism for concentrating urea before it is excreted is essential to the economy of the body fluid when water is in short supply.
When there is excess water in the body and low levels of ADH, the inner medullary collecting ducts have a much lower permeability to both water and urea, and more urea is excreted in the urine.
Countercurrent Exchange in the Vasa Recta Preserves Hyperosmolarity of the Renal Medulla
Blood flow must be provided to the renal medulla to supply the metabolic needs of the cells in this part of the kidney. Without a special medullary blood flow system, the solutes pumped into the renal medulla by the countercurrent multiplier system would be rapidly dissipated.
There are two special features of the renal medullary blood flow that contribute to the preserva-tion of the high solute concentrations:
1. The medullary blood flow is low, accounting forless than 5 per cent of the total renal blood flow. This sluggish blood flow is sufficient to supply the metabolic needs of the tissues but helps to minimize solute loss from the medullary interstitium.
2. The vasa recta serve as countercurrent exchangers,minimizing washout of solutes from the medullary interstitium.
The countercurrent exchange mechanism operates as follows (Figure 28–6): Blood enters and leaves the medulla by way of the vasa recta at the boundary of the cortex and renal medulla. The vasa recta, like other capillaries, are highly permeable to solutes in the blood, except for the plasma proteins. As blood descends into the medulla toward the papillae, it becomes progressively more concentrated, partly by solute entry from the interstitium and partly by loss of water into the interstitium. By the time the blood reaches the tips of the vasa recta, it has a con-centration of about 1200 mOsm/L, the same as that of the medullary interstitium. As blood ascends back toward the cortex, it becomes progressively less concentrated as solutes diffuse back out into the medullary interstitium and as water moves into the vasa recta.
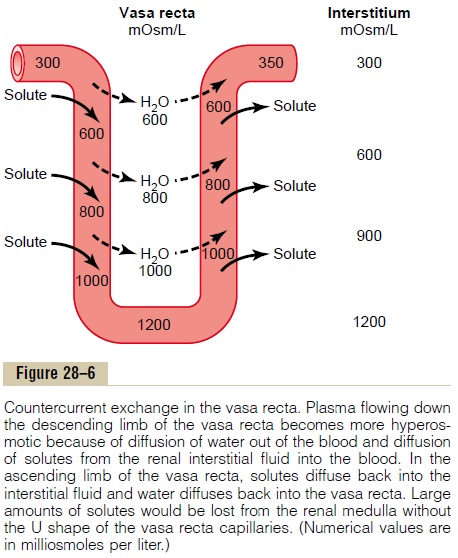
Thus, although there is a large amount of fluid and solute exchange across the vasa recta, there is little net dilution of the concentration of the interstitial fluid at each level of the renal medulla because of the U shape of the vasa recta capillaries, which act as countercur-rent exchangers. Thus, the vasa recta do not create themedullary hyperosmolarity, but they do prevent it from being dissipated.
The U-shaped structure of the vessels minimizes loss of solute from the interstitium but does not prevent the bulk flow of fluid and solutes into the blood through the usual colloid osmotic and hydro-static pressures that favor reabsorption in these capil-laries. Thus, under steady-state conditions, the vasa recta carry away only as much solute and water as is absorbed from the medullary tubules, and the high concentration of solutes established by the counter-current mechanism is maintained.
Increased Medullary Blood Flow Can Reduce Urine Concentrat-ing Ability. Certain vasodilators can markedly increaserenal medullary blood flow, thereby “washing out” some of the solutes from the renal medulla and reduc-ing maximum urine concentrating ability. Large increases in arterial pressure can also increase the blood flow of the renal medulla to a greater extent than in other regions of the kidney and tend to wash out the hyperosmotic interstitium, thereby reducing urine concentrating ability. As discussed earlier, maximum concentrating ability of the kidney is deter-mined not only by the level of ADH but also by the osmolarity of the renal medulla interstitial fluid. Even with maximal levels of ADH, urine concentrating ability will be reduced if medullary blood flow increases enough to reduce the hyperosmolarity in the renal medulla.
Summary of Urine Concentrating Mechanism and Changes in Osmolarity in Different Segments of the Tubules
The changes in osmolarity and volume of the tubular fluid as it passes through the different parts of the nephron are shown in Figure 28–7.
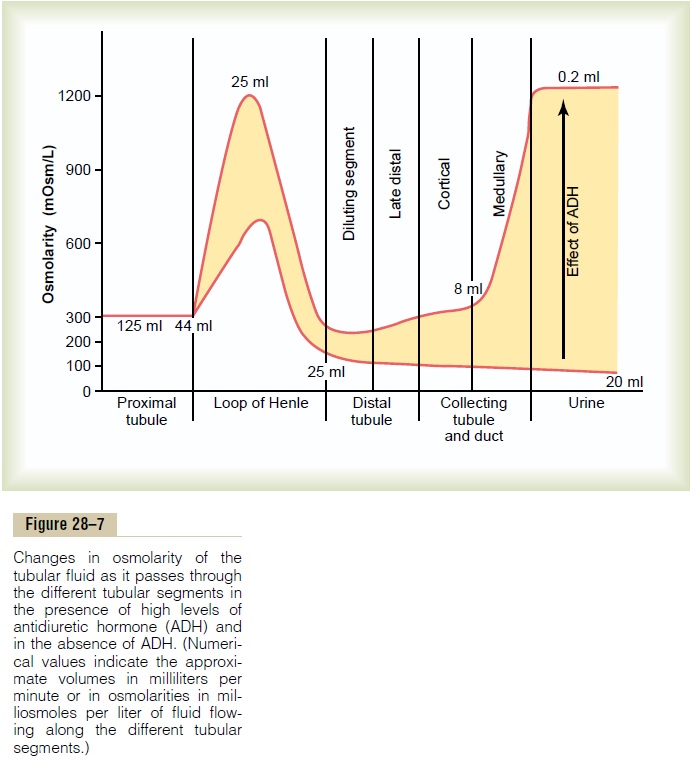
Proximal Tubule. About 65 per cent of thefilteredelectrolytes are reabsorbed in the proximal tubule.
However, the tubular membranes are highly perme-able to water, so that whenever solutes are reabsorbed, water also diffuses through the tubular membrane by osmosis. Therefore, the osmolarity of the fluid remains about the same as the glomerular filtrate, 300 mOsm/L.
Descending Loop of Henle. Asfluidflows down thedescending loop of Henle, water is absorbed into the medulla. The descending limb is highly permeable to water but much less permeable to sodium chloride and urea. Therefore, the osmolarity of the fluid flowing through the descending loop gradually increases until it is equal to that of the surrounding interstitial fluid, which is about 1200 mOsm/L when the blood concen-tration of ADH is high. When a dilute urine is being formed, owing to low ADH concentrations, the med-ullary interstitial osmolarity is less than 1200 mOsm/L; consequently, the descending loop tubular fluid osmo-larity also becomes less concentrated. This is due partly to the fact that less urea is absorbed into the medullary interstitium from the collecting ducts when ADH levels are low and the kidney is forming a large volume of dilute urine.
Thin Ascending Loop of Henle. The thin ascending limb isessentially impermeable to water but reabsorbs some sodium chloride. Because of the high concentration of sodium chloride in the tubular fluid, owing to water removal from the descending loop of Henle, there is some passive diffusion of sodium chloride from the thin ascending limb into the medullary interstitium. Thus, the tubular fluid becomes more dilute as the sodium chloride diffuses out of the tubule and water remains in the tubule. Some of the urea absorbed into the medullary interstitium from the collecting ducts also diffuses into the ascending limb, thereby return-ing the urea to the tubular system and helping to prevent its washout from the renal medulla. This urearecycling is an additional mechanism that contributesto the hyperosmotic renal medulla.
Thick Ascending Loop of Henle. The thick part of theascending loop of Henle is also virtually impermeable to water, but large amounts of sodium, chloride, potas-sium, and other ions are actively transported from the tubule into the medullary interstitium. Therefore, fluid in the thick ascending limb of the loop of Henle becomes very dilute, falling to a concentration of about 100 mOsm/L.
Early Distal Tubule. The early distal tubule has proper-ties similar to those of the thick ascending loop of Henle, so that further dilution of the tubular fluid occurs as solutes are reabsorbed while water remains in the tubule.
Late Distal Tubule and Cortical Collecting Tubules. In the latedistal tubule and cortical collecting tubules, the osmo-larity of the fluid depends on the level of ADH. With high levels of ADH, these tubules are highly perme-able to water, and significant amounts of water are reabsorbed. Urea, however, is not very permeant in this part of the nephron, resulting in increased urea concentration as water is reabsorbed. This allows most of the urea delivered to the distal tubule and collect-ing tubule to pass into the inner medullary collecting ducts, from which it is eventually reabsorbed or excreted in the urine. In the absence of ADH, little water is reabsorbed in the late distal tubule and corti-cal collecting tubule; therefore, osmolarity decreases even further because of continued active reabsorption of ions from these segments.
Inner Medullary Collecting Ducts. The concentration of fluid in the inner medullary collecting ducts also depends on (1) ADH and (2) the osmolarity of the medullary interstitium established by the countercur-rent mechanism. In the presence of large amounts of ADH, these ducts are highly permeable to water, and water diffuses from the tubule into the interstitium until osmotic equilibrium is reached, with the tubular fluid having about the same concentration as the renal medullary interstitium (1200 to 1400 mOsm/L). Thus, a very concentrated but small volume of urine is pro-duced when ADH levels are high. Because water reab-sorption increases urea concentration in the tubular fluid, and because the inner medullary collecting ducts have specific urea transporters that greatly facilitate diffusion, much of the highly concentrated urea in the ducts diffuses out of the tubular lumen into the medullary interstitium. This absorption of the urea into the renal medulla contributes to the high osmo-larity of the medullary interstitium and the high con-centrating ability of the kidney.
There are several important points to consider that may not be obvious from this discussion. First, although sodium chloride is one of the principal solutes that contributes to the hyperosmolarity of the medullary interstitium, the kidney can, when needed, excrete a highly concentrated urine that contains little sodium chloride. The hyperosmolarity of the urine inthese circumstances is due to high concentrations of other solutes, especially of waste products such as urea and creatinine. One condition in which this occurs is dehydration accompanied by low sodium intake. As discussed, low sodium intake stimulates formation of the hormones angiotensin II and aldos-terone, which together cause avid sodium reabsorption from the tubules while leaving the urea and other solutes to maintain the highly concentrated urine.
Second, large quantities of dilute urine can beexcreted without increasing the excretion of sodium.This is accomplished by decreasing ADH secretion, which reduces water reabsorption in the more distal tubular segments without significantly altering sodium reabsorption.
And finally, we should keep in mind that there is an obligatory urine volume, which is dictated by the maximum concentrating ability of the kidney and the amount of solute that must be excreted. Therefore, if large amounts of solute must be excreted, they must be accompanied by the minimal amount of water necessary to excrete them. For example, if 1200 mil-liosmoles of solute must be excreted each day, this requires at least 1 liter of urine if maximal urine con-centrating ability is 1200 mOsm/L.
Related Topics