Chapter: Medical Physiology: The Eye: II. Receptor and Neural Function of the Retina
Rhodopsin Retinal Visual Cycle, and Excitation of the Rods - Photochemistry of Eye Vision
Rhodopsin-Retinal Visual Cycle, and Excitation of the Rods
Rhodopsin and Its Decomposition by Light Energy. The outersegment of the rod that projects into the pigment layer of the retina has a concentration of about 40 per cent of the light-sensitive pigment called rhodopsin, or visual purple. This substance is a combination of theprotein scotopsin and the carotenoid pigment retinal (also called “retinene”). Furthermore, the retinal is a particular type called 11-cis retinal. This cis form of retinal is important because only this form can bind with scotopsin to synthesize rhodopsin.
When light energy is absorbed by rhodopsin, the rhodopsin begins to decompose within a very small fraction of a second, as shown at the top of Figure 50–5. The cause of this is photoactivation of electrons in the retinal portion of the rhodopsin, which leads to instantaneous change of the cis form of retinal into an all-trans form that still has the same chemical structure as the cis form but has a different physical structure— a straight molecule rather than an angulated molecule. Because the three-dimensional orientation of the reactive sites of the all-trans retinal no longer fits with the orientation of the reactive sites on the protein scotopsin, the all-transretinal begins to pullaway from the scotopsin. The immediate product is bathorhodopsin, which is a partially split combinationof the all-trans retinal and scotopsin. Bathorhodopsin
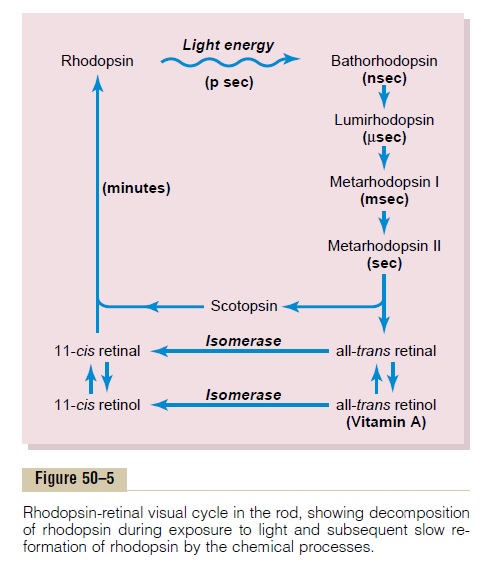
is extremely unstable and decays in nanoseconds to lumirhodopsin. This then decays in microsecondsto metarhodopsin I, then in about a millisecond to metarhodopsin II, and finally, much more slowly (inseconds), into the completely split products scotopsin and all-trans retinal.
It is the metarhodopsin II, also called activatedrhodopsin, that excites electrical changes in the rods,and the rods then transmit the visual image into the central nervous system in the form of optic nerve action potential, as we discuss later.
Re-formation of Rhodopsin. The first stage in re-formationof rhodopsin, as shown in Figure 50–5, is to reconvert the all-trans retinal into 11-cis retinal. This process requires metabolic energy and is catalyzed by the enzyme retinal isomerase. Once the 11-cis retinal is formed, it automatically recombines with the scotopsin to re-form rhodopsin, which then remains stable until its decomposition is again triggered by absorption of light energy.
Role of Vitamin A for Formation of Rhodopsin. Note inFigure 50–5 that there is a second chemical route by which all-trans retinal can be converted into 11-cis retinal.This is by conversion of the all-trans retinal first into all-trans retinol, which is one form of vitamin A. Then the all-trans retinol is converted into 11-cis retinol under the influence of the enzyme isomerase. Finally, the 11-cis retinol is converted into 11-cis retinal, which combines with scotopsin to form new rhodopsin.
Vitamin A is present both in the cytoplasm of the rods and in the pigment layer of the retina. Therefore, vitamin A is normally always available to form new retinal when needed. Conversely, when there is excess retinal in the retina, it is converted back into vitamin A, thus reducing the amount of light-sensitive pigment in the retina. We shall see later that this interconver-sion between retinal and vitamin A is especially impor-tant in long-term adaptation of the retina to different light intensities.
Night Blindness. Night blindness occurs in any personwith severe vitamin A deficiency. The simple reason for this is that without vitamin A, the amounts of retinal and rhodopsin that can be formed are severely depressed. This condition is called night blindnessbecause the amount of light available at night is too little to permit adequate vision in vitamin A–deficient persons.
For night blindness to occur, a person usually must remain on a vitamin A–deficient diet for months, because large quantities of vitamin A are normally stored in the liver and can be made available to the eyes. Once night blindness develops, it can sometimes be reversed in less than 1 hour by intravenous injection of vitamin A.
Excitation of the Rod When Rhodopsin Is Activated by Light
The Rod Receptor Potential Is Hyperpolarizing, Not Depolariz-ing. When the rod is exposed to light, the resulting receptor potential is different from the receptor poten-tials in almost all other sensory receptors. That is, exci-tation of the rod causes increased negativity of the intrarod membrane potential, which is a state of hyper-polarization, meaning that there is more negativitythan normal inside the rod membrane. This is exactly opposite to the decreased negativity (the process of “depolarization”) that occurs in almost all other sensory receptors.
But how does activation of rhodopsin cause hyper-polarization? The answer is that when rhodopsindecomposes, it decreases the rod membrane conduc-tance for sodium ions in the outer segment of the rod.
This causes hyperpolarization of the entire rod mem-brane in the following way.
Figure 50–6 shows movement of sodium ions in a complete electrical circuit through the inner and outer segments of the rod. The inner segment continually pumps sodium from inside the rod to the outside, thereby creating a negative potential on the inside of the entire cell. However, the outer segment of the rod, where the photoreceptor discs are located, is entirely different; here, the rod membrane, in the dark state, is very leaky to sodium ions. Therefore, positively charged sodium ions continually leak back to the inside of the rod and thereby neutralize much of the negativity on the inside of the entire cell. Thus, undernormal dark conditions, when the rod is not excited, there is reduced electronegativity inside the membraneof the rod, measuring about –40 millivolts rather than the usual –70 to –80 millivolts found in most sensory receptors.
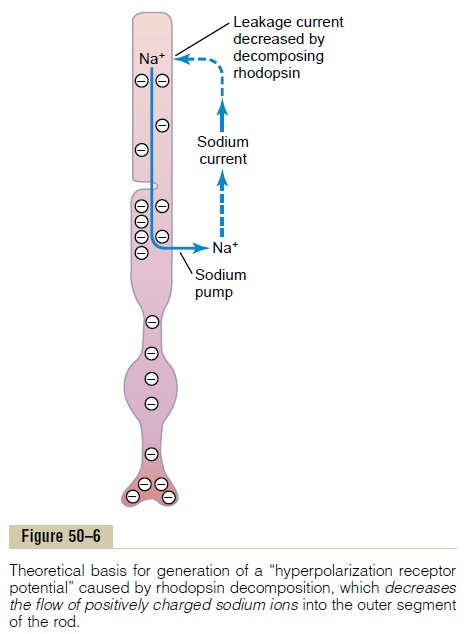
Then, when the rhodopsin in the outer segment of the rod is exposed to light, the rhodopsin begins to decompose, and this decreasesthe outer segment membrane conductance of sodium to the interior of the rod, even though sodium ions continue to be pumped outward through the membrane of the inner segment. Thus, more sodium ions now leave the rod than leak back in. Because they are positive ions, their loss from inside the rod creates increased negativity inside the membrane, and the greater the amount of light energy striking the rod, the greater the elec-tronegativity becomes—that is, the greater is the degree of hyperpolarization. At maximum light inten-sity, the membrane potential approaches –70 to –80 millivolts, which is near the equilibrium potential for potassium ions across the membrane.
Duration of the Receptor Potential, and Logarithmic Relation of the Receptor Potential to Light Intensity. When a sudden pulse of light strikes the retina, the transient hyperpolarization that occurs in the rods— that is, the receptor potential that occurs—reaches a peak in about 0.3 second and lasts for more than a second. In cones, the change occurs four times as fast as in the rods. A visual image impinged on the rods of the retina for only one millionth of a second can sometimes cause the sensation of seeing the image for longer than a second.
Another characteristic of the receptor potential is that it is approximately proportional to the logarithm of the light intensity. This is exceedingly important, because it allows the eye to discriminate light intensi-ties through a range many thousand times as great as would be possible otherwise.
Mechanism by Which Rhodopsin Decomposition Decreases Membrane Sodium Conductance—TheExcitation “Cascade.” Under optimal conditions, a single photon of light, the smallest possible quantal unit of light energy, can cause a measurable receptor potential in a rod of about 1 millivolt. Only 30 photons of light will cause half saturation of the rod. How can such a small amount of light cause such great excitation? The answer is that the photoreceptors have an extremely sensitive chemical cascade that amplifies the stimulatory effects about a millionfold, as follows:
1.The photon activates an electron in the 11-cis retinal portion of the rhodopsin; this leads to the formation of metarhodopsin II,which is the active form of rhodopsin, as already discussed and shown in Figure 50–5.
2.The activated rhodopsin functions as an enzyme to activate many molecules of transducin, a protein present in an inactive form in the membranes of the discs and cell membrane of the rod.
3.The activated transducin activates many more molecules of phosphodiesterase.
4.Activated phosphodiesterase is another enzyme;it immediately hydrolyzes many molecules of cyclic guanosine monophosphate (cGMP), thusdestroying it. Before being destroyed, the cGMP had been bound with the sodium channel protein of the rod’s outer membrane in a way that “splints” it in the open state. But in light, when phosphodiesterase hydrolyzes the cGMP, this removes the splinting and allows the sodium channels to close. Several hundred channels close for each originally activated molecule of rhodopsin. Because the sodium flux through each of these channels has been extremely rapid, flow of more than a million sodium ions is blocked by the channel closure before the channel opens again. This diminution of sodium ion flow is what excites the rod, as already discussed.
5.Within about a second, another enzyme, rhodopsin kinase, which is always present inthe rod, inactivates the activated rhodopsin (the metarhodopsin II), and the entire cascade reverses back to the normal state with open sodium channels.
Thus, the rods have developed an important chemi-cal cascade that amplifies the effect of a single photon of light to cause movement of millions of sodium ions. This explains the extreme sensitivity of the rods under dark conditions.
The cones are about 30 to 300 times less sensitive than the rods, but even this allows color vision at any intensity of light greater than extremely dim twilight.
Photochemistry of Color Vision by the Cones
It was pointed out at the outset of this discussion that the photochemicals in the cones have almost exactly the same chemical composition as that of rhodopsin in the rods. The only difference is that the protein por-tions, or the opsins—called photopsins in the cones— are slightly different from the scotopsin of the rods. The retinal portion of all the visual pigments is exactly the same in the cones as in the rods. The color-sensitive pigments of the cones, therefore, are combi-nations of retinal and photopsins.
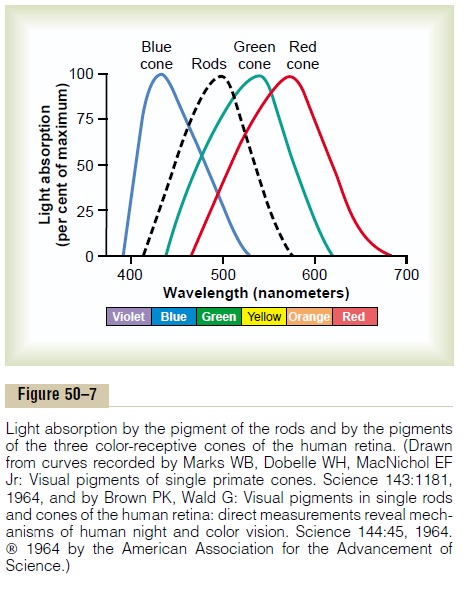
In the discussion of color vision later, it will become evident that only one of three types of color pigments is present in each of the different cones, thus making the cones selectively sensitive to different colors: blue, green, or red. These color pigments are called, respectively, blue-sensitive pigment, green-sensitive pigment, and red-sensitive pigment. Theabsorption characteristics of the pigments in the three types of cones show peak absorbencies at light wave-lengths of 445, 535, and 570 nanometers, respectively.
These are also the wavelengths for peak light sensitiv-ity for each type of cone, which begins to explain how the retina differentiates the colors. The approximate absorption curves for these three pigments are shown in Figure 50–7. Also shown is the absorption curve for the rhodopsin of the rods, with a peak at 505 nanometers.
Related Topics