Chapter: Medical Physiology: Regulation of Respiration
Respiratory Center
Transport of Carbon Dioxide in the Blood
Transport of carbon dioxide by the blood is not nearly as problematical as transport of oxygen is, because even in the most abnormal conditions, carbon dioxide can usually be transported in far greater quantities than oxygen can be. However, the amount of carbon dioxide in the blood has a lot to do with the acid-base balance of the body fluids. Under normal resting conditions, anaverage of 4 milliliters of carbon dioxide is transported from the tissues to the lungs in each 100 milliliters of blood.
Chemical Forms in Which Carbon Dioxide Is Transported
To begin the process of carbon dioxide transport, carbon dioxide diffuses out of the tissue cells in the dissolved molecular carbon dioxide form. On entering the tissue capillaries, the carbon dioxide initiates a host of almost instantaneous physical and chemical reac-tions, shown in Figure 40–13, which are essential for carbon dioxide transport.
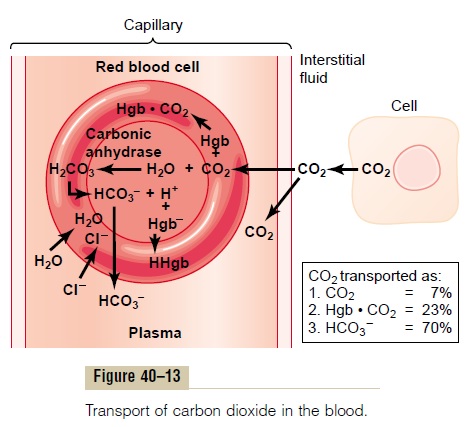
Transport of Carbon Dioxide in the Dissolved State. A smallportion of the carbon dioxide is transported in the dis-solved state to the lungs. Recall that the PCO2 of venous blood is 45 mm Hg and that of arterial blood is 40 mm Hg. The amount of carbon dioxide dissolved in the fluid of the blood at 45 mm Hg is about 2.7 ml/dl (2.7 volumes per cent). The amount dissolved at 40 mm Hg is about 2.4 milliliters, or a difference of 0.3 milliliter. Therefore, only about 0.3 milliliter of carbon dioxide is transported in the dissolved form by each 100 milliliters of blood flow. This is about 7 per cent of all the carbon dioxide normally transported.
Transport of Carbon Dioxide in the Form of Bicarbonate Ion Reaction of Carbon Dioxide with Water in the Red Blood Cells—Effect of Carbonic Anhydrase. The dis-solved carbon dioxide in the blood reacts with water to form carbonic acid. This reaction would occur much too slowly to be of importance were it not for the fact that inside the red blood cells is a protein enzyme called carbonic anhydrase, which catalyzes the reaction between carbon dioxide and water and accel-erates its reaction rate about 5000-fold. Therefore, instead of requiring many seconds or minutes to occur, as is true in the plasma, the reaction occurs so rapidly in the red blood cells that it reaches almost complete equilibrium within a very small fraction of a second. This allows tremendous amounts of carbon dioxide to react with the red blood cell water even before the blood leaves the tissue capillaries.
Dissociation of Carbonic Acid into Bicarbonate and Hydrogen Ions. In another fraction of a second, thecarbonic acid formed in the red cells (H2CO3) disso-ciates into hydrogen and bicarbonate ions (H+ and HCO3–). Most of the hydrogen ions then combine with the hemoglobin in the red blood cells, because the hemoglobin protein is a powerful acid-base buffer. In turn, many of the bicarbonate ions diffuse from the red cells into the plasma, while chloride ions diffuse into the red cells to take their place. This is made possible by the presence of a special bicarbonate-chloridecarrier protein in the red cell membrane that shuttlesthese two ions in opposite directions at rapid veloci-ties. Thus, the chloride content of venous red blood cells is greater than that of arterial red cells, a phe-nomenon called the chloride shift.
The reversible combination of carbon dioxide with water in the red blood cells under the influence of car-bonic anhydrase accounts for about 70 per cent of the carbon dioxide transported from the tissues to the lungs. Thus, this means of transporting carbon dioxide is by far the most important. Indeed, when a carbonic anhydrase inhibitor (acetazolamide) is administered to an animal to block the action of carbonic anhydrase in the red blood cells, carbon dioxide transport from the tissues becomes so poor that the tissue PCO2 can be made to rise to 80 mm Hg instead of the normal 45 mm Hg.
Transport of Carbon Dioxide in Combination with Hemoglobin and Plasma Proteins—Carbaminohemoglobin. In addition toreacting with water, carbon dioxide reacts directly with amine radicals of the hemoglobin molecule to form the compound carbaminohemoglobin(CO2Hgb). This combination of carbon dioxide and hemoglobin is a reversible reaction that occurs with a loose bond, so that the carbon dioxide is easily released into the alveoli, where the PCO2 is lower than in the pulmonary capillaries.
A small amount of carbon dioxide also reacts in the same way with the plasma proteins in the tissue capil-laries. This is much less significant for the transport of carbon dioxide because the quantity of these proteins in the blood is only one fourth as great as the quantity of hemoglobin.
The quantity of carbon dioxide that can be carried from the peripheral tissues to the lungs by carbamino combination with hemoglobin and plasma proteins is about 30 per cent of the total quantity transported— that is, normally about 1.5 milliliters of carbon dioxide in each 100 milliliters of blood. However, because this reaction is much slower than the reaction of carbon dioxide with water inside the red blood cells, it is doubtful that under normal conditions this carbamino mechanism transports more than 20 per cent of the total carbon dioxide.
Carbon Dioxide Dissociation Curve
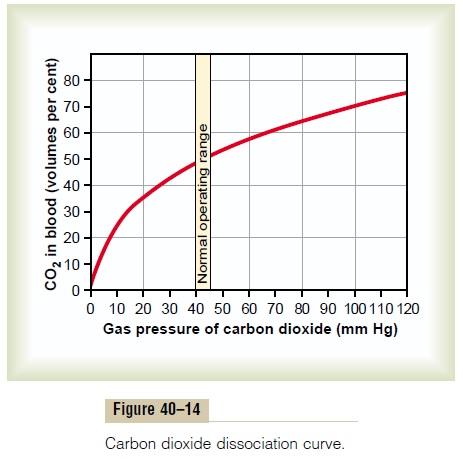
The curve shown in Figure 40–14—called the carbondioxide dissociation curve—depicts the dependenceof total blood carbon dioxide in all its forms on PCO2. Note that the normal blood PCO2 ranges between the limits of 40 mm Hg in arterial blood and 45 mm Hg in venous blood, which is a very narrow range. Note also that the normal concentration of carbon dioxide in the blood in all its different forms is about 50 volumes per cent, but only 4 volumes per cent of this is exchanged during normal transport of carbon dioxide from the tissues to the lungs. That is, the concentration rises to about 52 volumes per cent as the blood passes through the tissues and falls to about 48 volumes per cent as it passes through the lungs.
When Oxygen Binds with Hemoglobin, Carbon Dioxide Is Released (the Haldane Effect) to Increase CO2Transport
Earlier, it was pointed out that an increase in carbon dioxide in the blood causes oxygen to be displaced from the hemoglobin (the Bohr effect), which is an important factor in increasing oxygen transport. The reverse is also true: binding of oxygen with hemoglobin tends to displace carbon dioxide from the blood. Indeed, this effect, called the Haldaneeffect, is quantitatively far more important in promoting carbon dioxide transport than is the Bohr effect in promoting oxygen transport.
The Haldane effect results from the simple fact that the combination of oxygen with hemoglobin in the lungs causes the hemoglobin to become a stronger acid. This displaces carbon dioxide from the blood and into the alveoli in two ways: (1) The more highly acidic hemoglobin has less tendency to combine with carbon dioxide to form carbaminohemoglobin, thus displacing much of the carbon dioxide that is present in the car-bamino form from the blood. (2) The increased acidity of the hemoglobin also causes it to release an excess of hydrogen ions, and these bind with bicarbonate ions to form carbonic acid; this then dissociates into water and carbon dioxide, and the carbon dioxide is released from the blood into the alveoli and, finally, into the air.
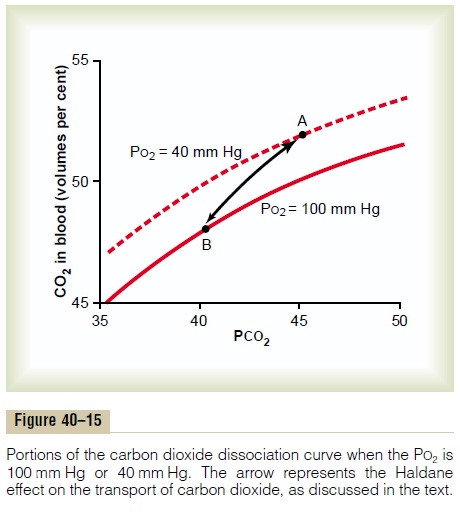
Figure 40–15 demonstrates quantitatively the signif-icance of the Haldane effect on the transport of carbon dioxide from the tissues to the lungs. This figure shows small portions of two carbon dioxide dissociation curves: (1) when the PO2 is 100 mm Hg, which is the case in the blood capillaries of the lungs, and (2) when the PO2 is 40 mm Hg, which is the case in the tissue cap-illaries. Point A shows that the normal PCO2 of 45 mm Hg in the tissues causes 52 volumes per cent of carbon dioxide to combine with the blood. On enter-ing the lungs, the PCO2 falls to 40 mm Hg and the PO2 rises to 100 mm Hg. If the carbon dioxide dissociation curve did not shift because of the Haldane effect, the carbon dioxide content of the blood would fall only to 50 volumes per cent, which would be a loss of only 2 volumes per cent of carbon dioxide. However, the increase in PO2 in the lungs lowers the carbon dioxide dissociation curve from the top curve to the lower curve of the figure, so that the carbon dioxide content falls to 48 volumes per cent (point B). This represents an additional 2 volumes per cent loss of carbon dioxide. Thus, the Haldane effect approximately doubles the amount of carbon dioxide released from the blood in the lungs and approximately doubles the pickup of carbon dioxide in the tissues.
Change in Blood Acidity During Carbon Dioxide Transport
The carbonic acid formed when carbon dioxide enters the blood in the peripheral tissues decreases the blood pH. However, reaction of this acid with the acid-base buffers of the blood prevents the hydrogen ion concen-tration from rising greatly (and the pH from falling greatly). Ordinarily, arterial blood has a pH of about 7.41, and as the blood acquires carbon dioxide in the tissue capillaries, the pH falls to a venous value of about 7.37. In other words, a pH change of 0.04 unit takes place. The reverse occurs when carbon dioxide is released from the blood in the lungs, with the pH rising to the arterial value of 7.41 once again. In heavy exer-cise or other conditions of high metabolic activity, or when blood flow through the tissues is sluggish, the decrease in pH in the tissue blood (and in the tissues themselves) can be as much as 0.50, about 12 times normal, thus causing significant tissue acidosis.
Related Topics