Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Cycles of activity and behavior
Reproductive seasonality - Seasonal patterns of Fishes
Reproductive seasonality
The most notably seasonal activity in fishes and most other organisms is reproduction. Successful reproduction requires careful synchrony in physiology, anatomy, and behavior of both sexes. Spawning occurs when both sexes have completed gametogenesis, gamete maturation, secondary sex character development, and spawning readiness and arrive at the proper spawning locale at the same time. A series of environmental cues are likely to trigger each stage of a reproductive cycle. Seasonally dependable cues, particularly ones that may insure survival of larvae (plankton blooms, sea temperature changes, alterations in currents) are the most likely cues to be used and are usually associated with seasonal, cyclical climatic events such as monsoonal rains, oceanic surface and upwelling currents (e.g., El Niños), and temperature cycles. Although environmental cues influence timing, flatfishes and seabass held under constant laboratory conditions still show predictable seasonality in their gonadal cycles, indicating an endogenous basis to reproductive cycles (Bye 1990).
Seasonal cycles occur in most families, but “seasons” are defined differently in temperate versus tropical habitats.
Most species in temperate latitudes spawn in spring or summer, a few in fall and winter. Conditions favoring larval growth and survival appear to be primary determinants of the phasing of reproduction. In temperate locales, spring and summer are times of maximal food productivity, and are also periods when protective vegetation is maximally available. Although many fishes in tropical and even subtropical regions breed year round (e.g., livebearers, numerous reef species), even these species show periods of peak reproductive activity that occur at relatively predictable times of the year.
Temperate freshwater fishes undergo reproductive cycles that are influenced strongly by changing photoperiod (day length) andtemperature. Because gametogenesis is a complicated and lengthy process, environmental conditions at the time of initiation of gametogenesis will be different from those in effect when spawning occurs. Hence different cues are used at different phases in the cycle. In salmonids, spawning time is heritable, occurring at the same time each year over a period of 2–6 weeks in a particular genetic strain (Scott 1990). However, timing may differ among stocks in geographically nearby rivers or even in different streams flowing into the same river, reflecting locally adapted genotypes (e.g., NRC 1996b; Stewart et al. 2002) (Fig. 23.6). The rhythm is circa-annual, endogenous, and entrained by environmental cues, primarily photoperiod,but can be modified by temperature. Salmonids
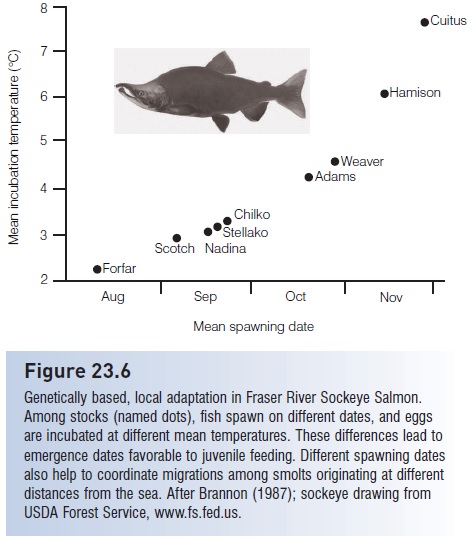
Figure 23.6
Genetically based, local adaptation in Fraser River Sockeye Salmon. Among stocks (named dots), fish spawn on different dates, and eggs are incubated at different mean temperatures. These differences lead to emergence dates favorable to juvenile feeding. Different spawning dates also help to coordinate migrations among smolts originating at different distances from the sea. After Brannon (1987); sockeye drawing from USDA Forest Service, www.fs.fed.us.
Most species are fall spawners, including Brown Trout, Brook Trout, Lake Trout, and Atlantic Salmon; among Pacific Oncorhynchus species, spawning occurs in late summer through early winter, with much latitudinal variation (e.g., Groot & Margolis 1991; Augerot 2005; Quinn 2005). Rainbow Trout are generally late winter spawners. In both groups, the reproductive cycle is initiated during the previous springtime in response to increasing day length.
Temperate cyprinids, such as the Golden Shiner (Notemigonus crysoleucas), Goldfish (Carassius auratus), Humpback Chub (Gila cypha), and Lake Chub (Couesius plumbeus), all spawn in late spring and early summer. Gametogenesis begins in the fall in response to decreasing temperature and shortening day length, advances slowly during the winter, and then accelerates and is completed in spring in response to increasing day length and rising temperature. Sticklebacks have a similar cycle, as do most spring/early summer spawning fishes in temperate locales. Carp (Cyprinus carpio) show a variant cycle, involving gonad development in late summer, quiescence in winter, and then final maturation of oocytes and spawning in the spring. The European Tench, Tinca tinca, is unusual in that it spawns in the fall. Late fall, winter, and early spring in temperate lakes are too unproductive for the small larvae of most species and consequently spawning does not normally occur during those seasons. The exceptionally large size of eggs and physiological tolerance of cold temperatures in salmonids may explain their fall–winter spawning and success at high latitudes (Baggerman 1990; Hontela & Stacey 1990).
Temperate fishes, as well as many other animals and plants, use photoperiod as a proximate environmental indicator of current and future climate. Typically, long days (e.g., >13 h light) and warm temperature cause gonadal recrudescence (resumption of gametogenic activity), whereas short days inhibit recrudescence, regardless of temperature.
Available evidence suggests that many temperate fish species have an endogenous, circa-annual clock that drives reproductive activities, and that this clock is affected by another, circadian clock of photosensitivity. A critical piece of information is day length; days shorter than some minimum cause both initiation and cessation of reproductive behavior. The circadian clock can tell a fish if day length is increasing, but the fish must be most sensitive to light at a time of the day when daylight would indicate increasing day length (Fig. 23.7). Daylight during the first 8–10 h after sunrise could occur at just about any time of year, but daylight 10–12 h or more after sunrise will not occur during winter. Hence fish have a clock that tells them how many hours have passed since sunrise, and they tend to be insensitive to light during the first 10 h or so after sunrise. Light encountered after that period, during the “photoinducible phase” of photosensitivity, has a strong influence on gonad development. The existence of a photoinducible phase and a photosensitive circadian rhythm was discovered by exposing fish to 2 h pulses of light at different times of the day. Sticklebacks exposed to light 14–16 h after sunrise showed greater rates of sexual maturation than fish experiencing light at other times of a light– dark cycle. The position and length of the photoinducible phase change with season and temperature (Baggerman 1990; Taylor 1990).
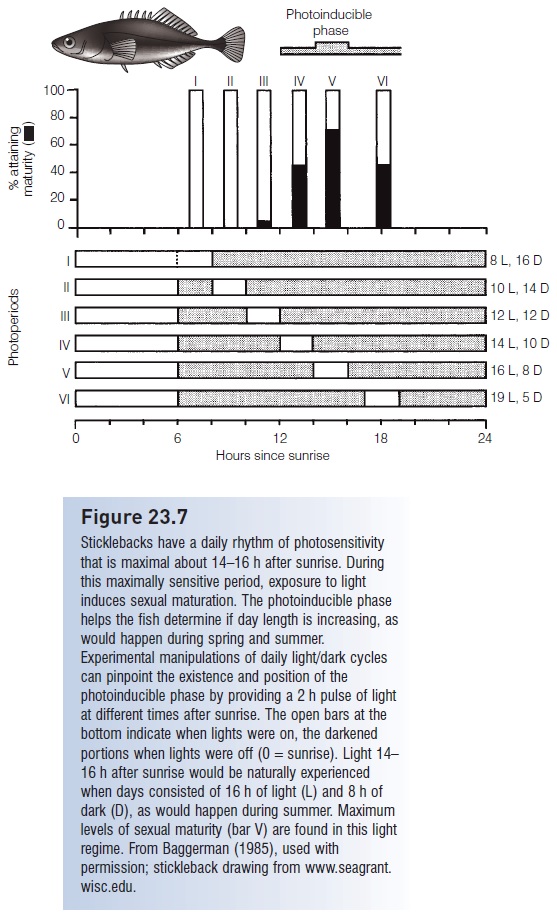
Figure 23.7
Sticklebacks have a daily rhythm of photosensitivity that is maximal about 14–16 h after sunrise. During this maximally sensitive period, exposure to light induces sexual maturation. The photoinducible phase helps the fish determine if day length is increasing, as would happen during spring and summer. Experimental manipulations of daily light/dark cycles can pinpoint the existence and position of the photoinducible phase by providing a 2 h pulse of light at different times after sunrise. The open bars at the bottom indicate when lights were on, the darkened portions when lights were off (0 =sunrise). Light 14– 16 h after sunrise would be naturally experienced when days consisted of 16 h of light (L) and 8 h of dark (D), as would happen during summer. Maximum levels of sexual maturity (bar V) are found in this light regime. From Baggerman (1985), used with permission; stickleback drawing from www.seagrant. wisc.edu.
Seasonality among freshwater fishes at tropical latitudes (between 30°N and 30°S latitude) is defined more by rainfall than by temperature (Goulding 1980; Lowe- McConnell 1987; Munro 1990a; Winemiller & Jepsen 1998) (Fig. 23.8). Regions between 15° north and south of the equator generally have two rainy seasons per year, whereas higher tropical latitudes have one rainy and one dry season. The floodplains, lakes, and seasonal swamps created by a rising river are common spawning and nursery grounds in many locales, and many riverine fishes have reproductive cycles that coincide with seasonal inundation of gallery forests and swamps, perhaps cued by rainfall or rising water levels (Lim et al. 1999; Agostinho et al. 2004; de Lima & Araujo-Lima 2004). Newly inundated areas are advantageous spawning locales because: (i) accumulated nutrients are released, which creates plankton blooms and food for progeny; and (ii) predation is minimized by abundant vegetation for refuging and because large flooded expanses minimize contact with predators. In contrast, receding water and reduced habitat space during the dry season means that predators and competitors are concentrated, which also leads to deoxygenated water. Dry season and aseasonal spawners often provide extensive parental care, including provisioning of young, and/or possess secondary breathing structures (e.g., lungfishes, bagrid catfishes, cichlids, anabantoids).
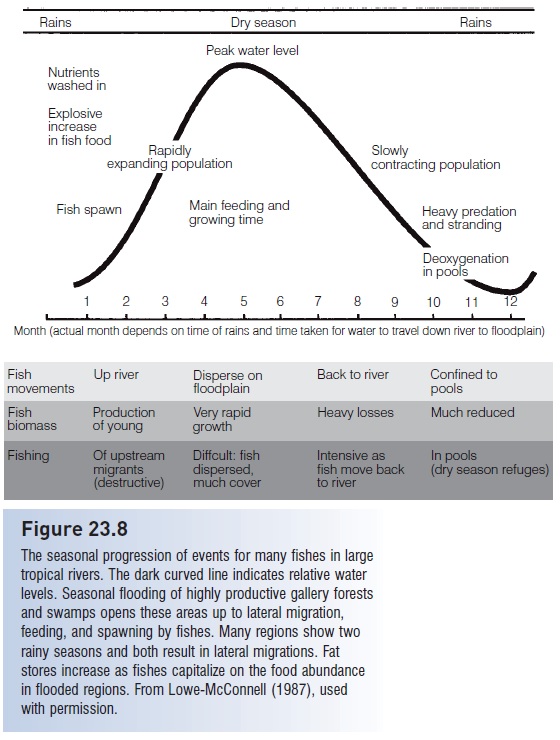
Figure 23.8
The seasonal progression of events for many fishes in large tropical rivers. The dark curved line indicates relative water levels. Seasonal flooding of highly productive gallery forests and swamps opens these areas up to lateral migration, feeding, and spawning by fishes. Many regions show two rainy seasons and both result in lateral migrations. Fat stores increase as fishes capitalize on the food abundance in flooded regions. From Lowe-McConnell (1987), used with permission.
Adults of many tropical freshwater species, including osteoglossid arapaima, mormyrids, large characins, cyprinids, many catfishes, and gymnotid knifefishes, migrate up tributaries and onto flooded plains to spawn (Fig. 23.8); many such migrations cover much more than 100 km (Munro 1990a; Lucas & Baras 2001; Welcomme 2003). Lake-dwelling species and populations in these families move into tributary streams, whereas lacustrine herrings, silversides, and percomorphs often spawn within the lake itself. Seasonality in other riverine species involves migrations upriver to headwater regions in anticipation of seasonal rains (e.g., large characins, catfishes). Many small characins, killifishes, livebearers, and cichlids reproduce year round, although peaks in recruitment often correspond with high water.
Worldwide, predatory species often spawn earlier than their prey, thus assuring a food source for young predators. For example, the South American characin, Hoplias malabaricus, is predatory throughout life and breeds earlier than most other species. In contrast, juvenile piranhas are omnivorous and adults breed along with other nonpredatory species, although predatory species that used to be restricted to seasonal spawning in inundated floodplains now regularly find favorable spawning habitat behind dams. Regardless, spawning migrations upriver or onto flooded areas must be preceded by gonadal recrudescence that anticipates seasonal rainfall by several months. The cues that stimulate gonadal growth and gametogenesis are poorly understood, but may include photoperiod and temperature changes (particularly at higher latitudes in the tropics), social interactions, food availability and energy stores, as well as endogenously controlled rhythms, perhaps entrained by previous spawning itself (Munro 1990a).
Temperate marine teleosts have restricted spawning periods that vary by species, locale, and genetic stock (Bye 1990). At any particular locale, however, a stock is likely to have a fairly short and predictable time period during which most spawning occurs. Typically, pelagic species spawn over a 4-month period, with a shorter period of maximal activity. For example, Cod in the North Sea off the northeast coast of England spawn between January and May, with 70% of eggs produced during 6 weeks of that period. Peak spawning of European Plaice (Pleuronectes platessa) in the Southern Bight of the North Sea occurred within 1 week of January 19 over the 39-year period between 1911 and 1950.
The locale of spawning is also fairly predictable and defined by oceanic phenomena such as thermoclines or frontal areas, which are transition regions between differing water masses. American and European eels migrate to spawn in a region of the Sargasso Sea where a persistent frontal zone, defined by marked horizontal differences in temperature, salinity, and water density, exists every spring (McCleave et al. 1987). Inshore, temperate marine species are strongly seasonal. Coastal California species spawn mostly in the spring and summer (e.g., Grunion, surfperches, halibut, Sheephead, Blacksmith, croakers, seabasses, pricklebacks), some spawn in winter or early spring (rockfishes, starry flounder, lingcod), and a few spawn in the fall (greenlings, cabezon). Predominantly spring and summer spawning also typifi es Atlantic tem perate fishes (halibut, killifishes, most flatfishes, seabasses, porgies, many croakers, wrasses), with a few winter spawners (Summer and Winter flounder, sculpins, some croakers) (Ferraro 1980; Holt et al. 1985).
Coral reefs experience less extreme seasonal variation than temperate habitats and are less subject to the vagaries of rainfall than tropical freshwater systems. As a consequence, many coral reef fishes spawn through most or all of the year, particularly at low latitudes (e.g., many damselfishes, wrasses, parrotfishes, grunts, surgeonfishes). Nevertheless, seasonal reproduction is also common among many families, including groupers, snappers, damselfishes, rabbitfishes, gobies, and pufferfishes. Seasonal spawning peaks have been found in most tropical locales, including sites in the Indian Ocean, South China Sea, and tropical Atlantic and Pacific oceans. “Springtime” peaks are most common, followed next by two periods of major spawning activity in the spring and fall. The most common environmental correlate of these peaks is that they occur when major currents around islands are weakest. Spawning during slack current periods would minimize the long-distance dispersal of larvae away from home reefs (Johannes 1978; Sale 1978; Robertson 1991).
Recruitment of larvae to the reef is also cyclical and seasonal. Even species that breed throughout the year show seasonal peaks in the arrival of larvae and episodic pulses of larval arrival. French Grunts, Haemulon flavolineatum, in the Caribbean breed year round. Larvae arrive in semilunar pulses over an 8-month period, with greatest recruitment in May, June, and November. Damselfishes in the southern Great Barrier Reef breed during a 5-month, summertime period and larvae are recruited in pulses, with one or a few major pulses accounting for most arrivals. Larvae arrive on a lunar cycle, the major feature being that little recruitment occurs around the time of the full moon. Most settlement of fish larvae on coral reefs occurs at night, suggesting a strong influence of visual predators. Avoidance of full moon periods would have a similar function. From the above, it is evident that spawning and recruitment do not necessarily follow the same timetables. Larvae can be produced, but conditions for settling after the larval period of a month or two may not be favorable. In fact, abundance of larvae offshore and settlement of larvae on the reef do not necessarily coincide. For example, Nassau Grouper, Epinephelus striatus, larvae can be found along the Bahamas Bank of the western Atlantic over a 2–3-month period, but actual larval settlement occurs almost entirely over a 4–6-night period when storm-driven currents push water and larvae into shore (McFarland et al. 1985; Doherty & Williams 1988; Doherty 1991; Shenker et al. 1993).
Related Topics