Chapter: Basic & Clinical Pharmacology : The Eicosanoids:Prostaglandins, Thromboxanes, Leukotrienes, & Related Compounds
Receptor Mechanisms - Mechanisms & Effects of Eicosanoids
BASIC
PHARMACOLOGY OF EICOSANOIDS
MECHANISMS & EFFECTS OF
EICOSANOIDS
Receptor Mechanisms
As
a result of their short half-lives, the eicosanoids act mainly in an autocrine
and a paracrine fashion, ie, close to the site of their synthesis, and not as
circulating hormones. These ligands bind to receptors on the cell surface, and
pharmacologic specificity is determined by receptor density and type on
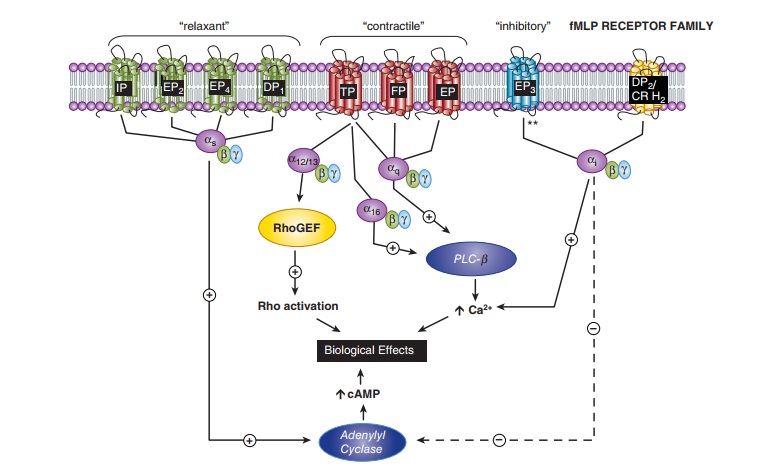
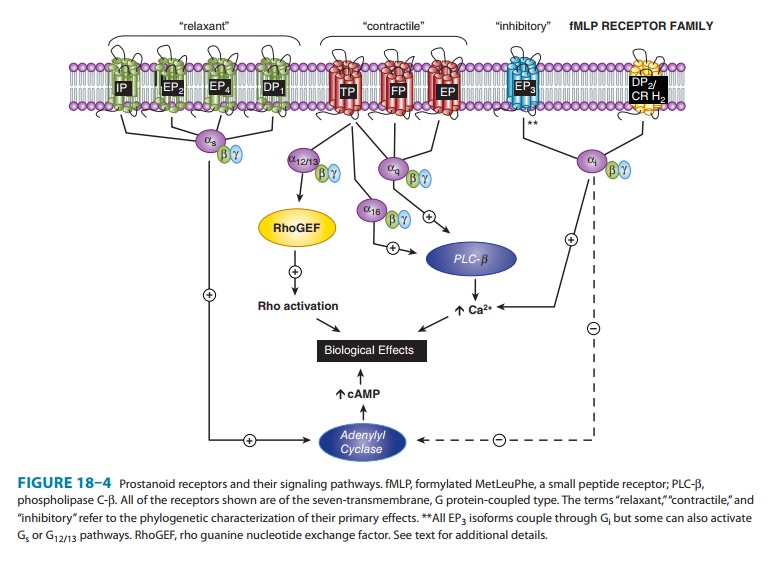
different cells (Figure 18–4). A single gene product has been identified for
the PGI2 (IP), PGF2α (FP), and TXA2 (TP) receptors,
while four dis-tinct PGE2 receptors (EPs 1–4) and two PGD2
receptors (DP1 and
DP2)
have been cloned. Additional isoforms of the human TP (α and β), FP (A and B), and EP3 (I, II, III,
IV, V, VI, e, and f ) recep-tors can arise through differential mRNA splicing.
Two receptors exist for both LTB4 (BLT1 and BLT2)
and the cysteinyl leukot-rienes (cysLT1 and cysLT2). The
formyl peptide (fMLP)-1 receptor can be activated by lipoxin A4 and
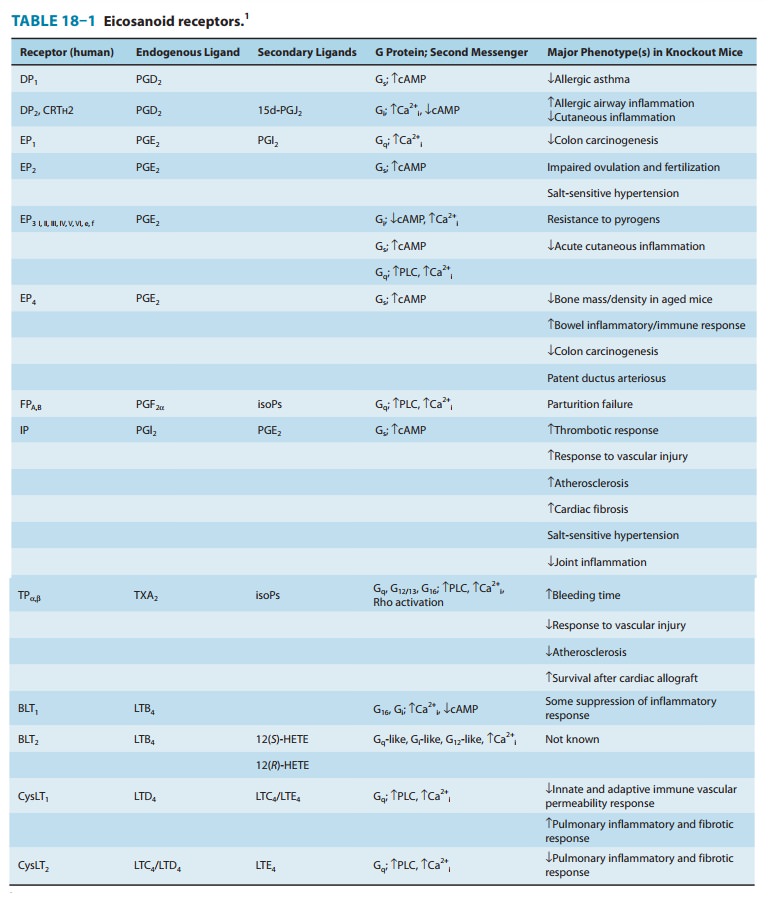
consequently has been termed the ALX receptor. All of these receptors are G
protein-coupled; properties of the best-studied receptors are listed in Table
18–1.
EP2,
EP4, IP, and DP1 receptors activate adenylyl cyclase via
Gs. This leads to increased intracellular cAMP levels, which in turn
activate specific protein kinases . EP1, FP, and TP activate
phosphatidylinositol metabolism, leading to the forma-tion of inositol
trisphosphate, with subsequent mobilization of Ca2+ stores and an
increase of free intracellular Ca2+. TP also couples to multiple G
proteins, including G12/13 and G16, to stimulate small G
protein signaling pathways, and may activate or inhibit adenylyl cyclase via Gs
(TPα)
or Gi (TPβ),
respectively. EP3 isoforms can couple to both elevation of
intracellular calcium and to increased or decreased cAMP. The DP 2
receptor (also known as the chemoattractant receptor-homologous molecule
expressed on TH2 cells, or CRTH2), which is unrelated to the other prostanoid
receptors, is a member of the fMLP (formylated MetLeuPhe) receptor superfamily.
This receptor couples througha Gi-type G protein and leads to
inhibition of cAMP synthesis and increases in intracellular Ca2+ in
a variety of cell types.
LTB4
also causes inositol trisphosphate release via the BLT1 receptor,
causing activation, degranulation, and superoxide anion generation in
leukocytes. The BLT2 receptor, a low-affinity recep-tor for LTB4,
is also bound with reasonable affinity by 12(S )- and 12(R )-HETE,
although the biologic relevance of this observation is not clear. CysLT1
and cysLT2 couple to Gq, leading to increased
intracellular Ca2+. Studies have also placed Gi
downstream of cysLT2. An orphan receptor, GPR17, binds cysLTs and
may nega-tively regulate the function of cysLT1, but its physiologic
role remains ill defined. As noted above, the EETs promote vasodila-tion via
paracrine activation of calcium-activated potassium chan-nels on smooth muscle
cells leading to hyperpolarization and relaxation. This occurs in a manner
consistent with activation of a Gs-coupled receptor, although a
specific EET receptor has yet to be identified. EETs may also act in an
autocrine manner directly activating endothelial transient receptor potential
channels to cause endothelial hyperpolarization, which is then transferred to
the smooth muscle cells by gap junctions or potassium ions. Specific receptors
for isoprostanes have not been identified, and the biologic importance of their
capacity to act as incidental ligands at prostaglandin receptors remains to be
established.
Although
prostanoids can activate peroxisome proliferator-activated receptors (PPARs) if
added in sufficient concentration in vitro, it remains questionable whether
these compounds attain concentrations sufficient to function as endogenous
nuclear-receptor ligands in vivo.
Related Topics