Chapter: Basic & Clinical Pharmacology : The Eicosanoids:Prostaglandins, Thromboxanes, Leukotrienes, & Related Compounds
Arachidonic Acid & Other Polyunsaturated Precursors
ARACHIDONIC ACID & OTHER
POLYUNSATURATED PRECURSORS
Arachidonic
acid (AA), or 5,8,11,14-eicosatetraenoic acid, the most abundant of the
eicosanoid precursors, is a 20-carbon (C20) fatty acid containing four double
bonds (designated C20:4–6).must first be released or mobilized from the sn-2
position of membrane phospholipids by one or more lipases of the phospho-lipase
A2 (PLA2) type (Figure 18–1) for eicosanoid synthesis to
occur. At least three classes of phospholipases mediate arachido-nate release
from membrane lipids: cytosolic (c) PLA2, secretory
PLA2,
and calcium-independent (i) PLA 2. Chemical and physical stimuli
activate the Ca 2+-dependent translocation of group IVA cPLA2,
which has high affinity for AA, to the mem-brane, where it releases
arachidonate. Multiple additional PLA2 isoforms (group VI iPLA2
and sPLA2 from groups IIA, V, and X) have been characterized. Under
nonstimulated conditions, AA liberated by iPLA 2 is reincorporated
into cell membranes, so there is negligible eicosanoid biosynthesis. While cPLA2
dominates in the acute release of AA, inducible sPLA2 contributes under
condi-tions of sustained or intense stimulation of AA production. AA can also
be released by a combination of phospholipase C and diglyceride lipase.
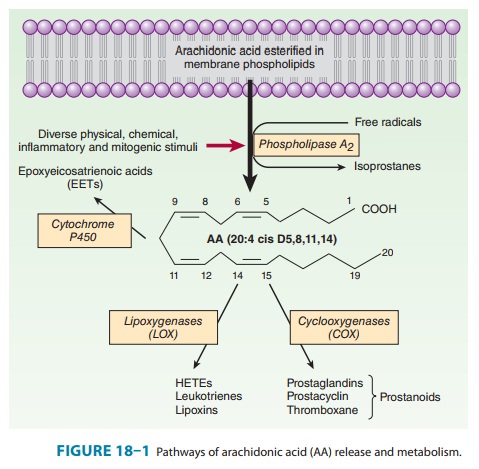
Following
mobilization, AA is oxygenated by four separate routes: the cyclooxygenase
(COX), lipoxygenase, P450 epoxyge-nase, and isoeicosanoid pathways (Figure
18–1). Among factors determining the type of eicosanoid synthesized are (1) the
sub-strate lipid species, (2) the type of cell, and (3) the manner in which the
cell is stimulated. Distinct but related products can be
ACRONYMS
AA Arachidonic
acid
COX Cyclooxygenase
DHET Dihydroxyeicosatrienoic
acid
EET Epoxyeicosatrienoic
acid
HETE Hydroxyeicosatetraenoic
acid
HPETE Hydroxyperoxyeicosatetraenoic
acid
LTB, LTC Leukotriene B,
C, etc
LOX Lipoxygenase
LXA, LXB Lipoxin A, B
NSAID Nonsteroidal
anti-inflammatory drug
PGE, PGF Prostaglandin
E, F, etc
PLA, PLC Phospholipase
A, C
TXA, TXB Thromboxane A,
B
formed
from precursors other than AA. For example, homo-γ-linoleic acid (C20:3–6) or eicosapentaenoic
acid (C20:5–3, EPA) yields products that differ quantitatively and
qualitatively from those derived from AA. This shift in product formation is
the basis for using fatty acids obtained from cold-water fish or from plants as
nutritional supplements in humans. For example, thromboxane (TXA 2),
a powerful vasoconstrictor and platelet agonist, is synthesized from AA via the
COX pathway. COX metabolism of EPA yields TXA 3, which is relatively
inactive. 3-Series prostaglandins, such as prostaglandin E3 (PGE3),
can also act as partial agonists or antagonists thereby reducing the activity
of their AA-derived 2-series counterparts. The hypothesis that dietary
eicosapentaenoate substitution for arachidonate could reduce the incidence of
cardiovascular disease and cancer is a focus of current investigation.
Related Topics