Chapter: Basic Radiology : Radiology of the Breast
Radiology of the Breast: Technique and Normal Anatomy
TECHNIQUE AND NORMAL ANATOMY
Film-screen and Digital Radiography (Radiomammography)
The film-screen mammogram is
created with x-rays, radi-ographic film, and intensifying screens adjacent to
the film within the cassette; hence the term film-screen mammogra-phy. The digital mammogram is created using a
similar sys-tem, but replacing the film and screen with a digital detector.
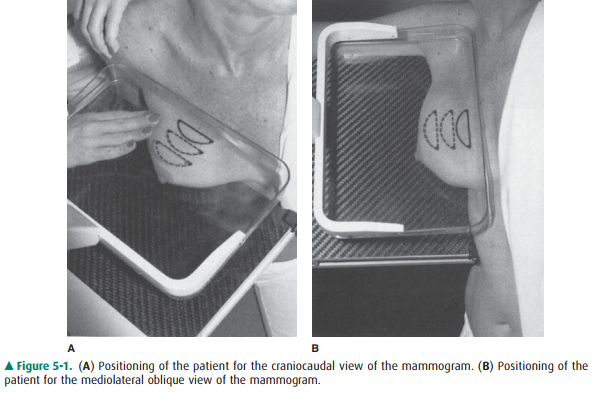
The routine examination consists
of two views of each breast, the craniocaudal (C-C) view and the mediolateral
oblique (MLO) view, with a total of four films. The C-C view can be considered
the “top-down” view, and the MLO an an-gled view from the side (Figures 5-1,
5-2). The patient un-dresses from the waist up and stands for the examination,
leaning slightly against the mammography unit. The technol-ogist must mobilize,
elevate, and pull the breast to place as much breast tissue as possible on the
surface of the film cas-sette holder. A flat, plastic compression paddle is
then gently but firmly lowered onto the breast surface to compress the breast
into as thin a layer as possible. This compression achieves both immobilization
during exposure and disper-sion of breast tissue shadows over a larger area,
thereby permitting better visual separation of imaged structures. Compression
may be uncomfortable, and may even be painful in a small proportion of
patients. However, most pa-tients accept this level of discomfort for the few
seconds re-quired for each exposure, particularly if they understand the need
for compression and know what to expect during the examination. Mammography has
proved to be more cost-effective, while maintaining resolution high enough to
demonstrate early malignant lesions, than any other breast im-aging technique.
In its present state of evolution, however, the sensitivity of radiomammography
ranges from 85% to 95%.
Limitations
Sensitivity is limited by three
factors: (1) the nature of breast parenchyma, (2) the difficulty in positioning
the organ for imaging, and (3) the nature of breast carcinoma.
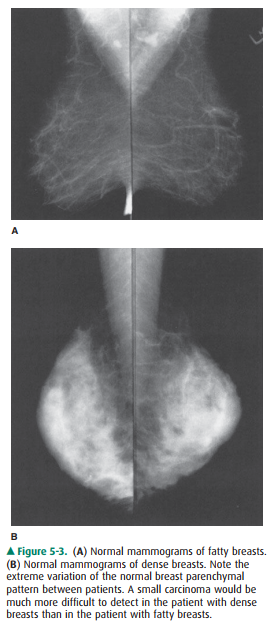
The Nature of Breast Parenchyma
Very dense breast tissue may
obscure masses lying within ad-jacent tissue. Masses are more easily detected
in a fatty breast.
Positioning
A technologist performing
mammography must include as much breast tissue as possible in the field of view
for each image. The x-ray beam must pass through the breast tangen-tially to
the thorax, and no other part of the body should in-trude into the field of
view, so as to not obscure any part of the breast. This requires both a
cooperative patient and a skilled technologist. If a breast mass is located in
a portion of the breast that is difficult to include in the image, mammog-raphy
may fail to demonstrate the lesion. Also, because of these practical considerations,
routine mammography is not performed in markedly debilitated patients.
The Nature of Breast Carcinoma
Some breast carcinomas are seen
as well-defined rounded masses or as tiny, but bright, calcifications, and are
easily de-tected. Others, however, may be poorly defined and irregular,
mimicking normal breast tissue. Rarely, still others may have no radiographic
signs at all.
For these reasons, it must be
remembered that mammog-raphy has significant limitations in detection of
carcinoma. It cannot be overemphasized that any suspicious finding on breast
physical examination should be evaluated further, even if the mammogram shows
no abnormality. Occasionally, ad-ditional imaging may reveal an abnormality,
but if not, short-term close clinical follow-up or biopsy is warranted.
Normal Structures
Normal breast is composed mainly
of parenchyma (lobules and ducts), connective tissue, and fat. Lobules are
drained by ducts, which arborize within lobes. There are about 15 to 20 lobes
in the breast. The lobar ducts converge upon the nipple.
Parenchyma
The lobules are glandular units
and are seen as ill-defined, splotchy opacities of medium density. Their size
varies from 1 to several millimeters, and larger opacities result from
con-glomerates of lobules with little interspersed fat. The breast lobes are
intertwined and are therefore not discretely identi-fiable. This parenchymal
tissue is contained between the pre-mammary and retromammary fascia.
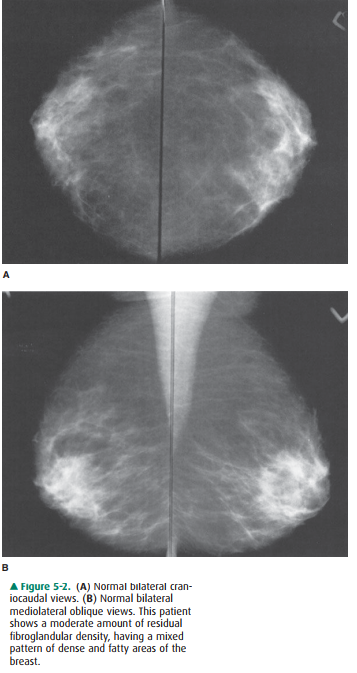
The amount and distribution of
glandular tissue are highly variable. Younger women tend to have more glandular
tissue than do older women. Glandular atrophy begins infer-omedially, and
residual glandular density persists longer in the upper outer breast quadrants.
However, any pattern can be seen at any adult age (Figure 5-3).
Along with glandular elements,
the parenchyma con-sists of ductal tissue. Only major ducts are visualized
mammographically, and these are seen in the subareolar re-gion as thickened
linear structures of medium density con-verging on the nipple.
Connective Tissue
Trabecular structures, which are
condensations of connec-tive tissue, appear as thin ( 1 mm) linear opacities of
medium to high density. Cooper’s ligaments are the sup-porting trabeculae over
the breast that give the organ its characteristic shape, and are thus seen as
curved lines around fat lobules along the skin-parenchyma interface within any
one breast (Figure 5-4).
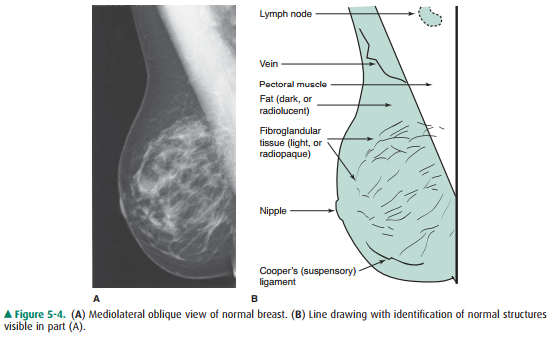
Fat
The breast is composed of a large
amount of fat, which is lu-cent, or almost black, on mammograms. Fat is
distributed in the subcutaneous layer, in among the parenchymal elements
centrally, and in the retromammary layer anterior to the pec-toral muscle
(Figure 5-4).
Lymph Nodes
Lymph nodes are seen in the
axillae and occasionally in the breast itself (Figure 5-4).
Veins
Veins are seen traversing the
breast as uniform, linear opaci-ties, about 1 to 5 mm in diameter (Figure 5-4).
Arteries
Arteries appear as slightly
thinner, uniform, linear densities and are best seen when calcified, as in
patients with athero-sclerosis, diabetes, or renal disease.
Skin
Skin lines are normally thin and
are not easily seen without the aid of a bright light for film-screen
mammograms. Vari-ous processing algorithms with digital mammography allow
better visualization of the skin.
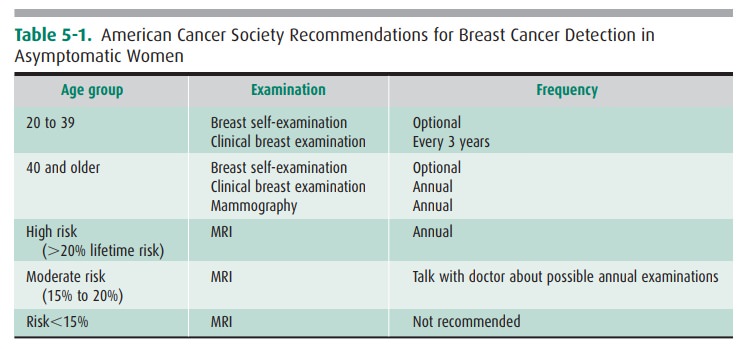
Screening Mammography
The standard mammogram (along
with appropriate history-taking) makes up the entire screening mammogram. The
indi-cation for this examination is the search for occult carcinoma in an
asymptomatic patient. Physical examination by the patient’s physician, known as
the clinical breast examination (CBE), is an indispensable element in complete
breast screening. Although the American Cancer Society no longer recommends
routine breast self-examination (BSE), particular attention should be paid to
lumps identified by the patient as new or enlarging. Such patients should be
referred for diagnostic mammography. Table 5-1 includes guidelines for
frequency.
Diagnostic Mammography
The diagnostic mammogram begins
with the two-view stan-dard mammogram. Additional maneuvers are then used
asappropriate in each case, dictated by history, physical exami-nation, and
findings on initial mammography. Indications for diagnostic mammography are (1)
a palpable mass or other symptom or sign (eg, skin dimpling, nipple retraction,
or nipple discharge that is clear or bloody), and (2) a radi-ographic
abnormality on a screening mammogram. Addi-tionally, patients with a personal
history of breast cancer may be considered in the diagnostic category.
Other projections, magnification,
and spot compression may be used to further evaluate abnormalities. These
tech-niques provide better detail and disperse overlapping breast tissue so
that lesions are less obscured.
Implant Views
Patients with breast implants
require specialized views to best image residual breast tissue because the
implants obscure large areas of the breast tissue with routine mammography.
These specialized views (Eklund, “push-back,” or implant displacement view)
displace the implants posteriorly while the breast tissue is pulled anteriorly
as much as possible.
Computer-Aided Detection
Growing availability and
affordability of computing power has led to the development of computer-aided
detection (CAD). CAD utilizes complex algorithms to analyze the data from a
mammogram for suspicious calcifications, masses, and architecture distortion.
It then flags these areas so that the interpreting radiologist can give these
areas special atten-tion. Several studies show increased cancer detection when
CAD is applied, and sensitivity and specificity continue to improve as these
algorithms are refined.
Ultrasonography
The indications for
ultrasonography are (1) a mammographi-cally detected mass, the nature of which
is indeterminate, (2) apalpable mass that is not seen on mammography, (3) a
palpa-ble mass in a patient below the age recommended for routine mammography,
and (4) guidance for intervention. Ultra-sonography is a highly reliable
technique for differentiating cystic from solid masses. If criteria for a
simple cyst are met, the diagnosis is over 99% accurate. Although certain
features have been described as indicative of benign or malignant solid masses,
this determination is more difficult to make and less accurate than the
determination of the cystic nature of a mass.
A limitation of ultrasonography
is that it is very operator-dependent. Also, it images only a small part of the
breast at any one moment. Therefore, an overall inclusive survey is not
possible in one image, and lesions may easily be missed.
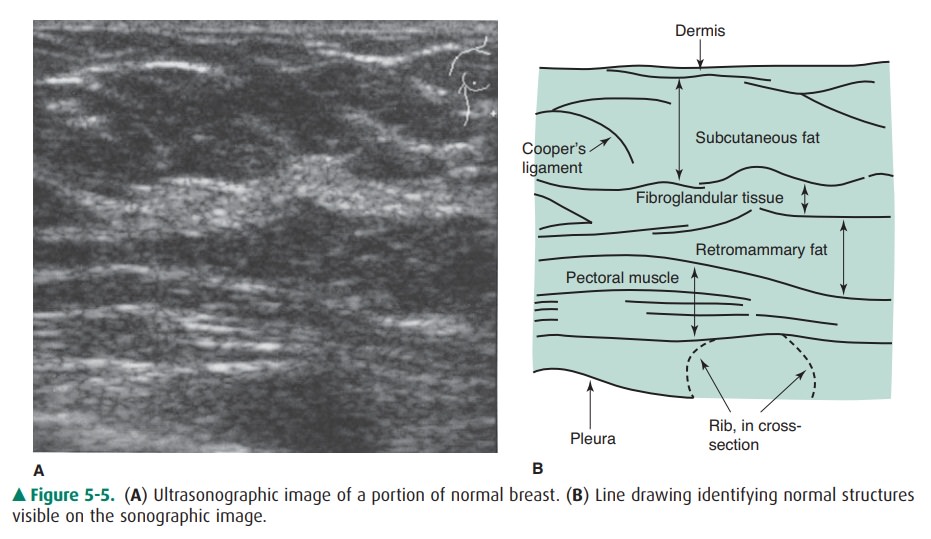
Normal Structures
The skin, premammary and
retromammary fasciae, trabecu-lae, walls of ducts and vessels, and pectoral
fasciae are well seen as linear structures. The glandular and fat lobules are
oval, of varying sizes, and hypoechoic relative to the sur-rounding connective
tissue (Figure 5-5).
Simple cysts are anechoic
(echo-free) and have thin, smooth walls. Increased echogenicity is seen deep to
cysts (enhanced through-transmission). Most solid masses are hy-poechoic
relative to surrounding breast tissue.
Magnetic Resonance Imaging
The role of MRI in mammography
continues to expand, with common applications including (1) staging of and
surgical planning for breast tumors, (2) searching for a primary tumor in
patients who present with cancerous axillary lymph nodes,evaluating tumor
response to neoadjuvant chemotherapy,differentiating tumor recurrence from
posttreatment changes in patients with previous breast-conserving surgery and
radiation, (5) screening of high-risk patients, (6) evaluating implants, and
(7) evaluating difficult (dense or fibrous) breasts. In addition, the
technology for MR-guided breast biopsies is in-creasingly available.
The patient lies prone on the
scanner table, and a special-ized coil surrounds the breasts. Depending on the
clinical question, a varying number of pulse sequences are performed to
evaluate the breasts or the composition of a suspicious le-sion. Scan times can
range from 30 minutes to over an hour.
MRI can show whether a lesion is
solid or contains fat or fluid. Dynamic scanning after administration of
intravenous contrast shows whether structures enhance and at what rate. Cancers
classically enhance rapidly with subsequent “wash-out.” For instance, a lesion
that enhances relatively rapidly on dynamic exam (think neovascularity) is more
concerning for malignancy. If more than one suspicious lesion is identi-fied,
the relative proximity of these lesions can determine whether a patient would
be a good candidate for lumpec-tomy rather than mastectomy. The wide field of
view allows staging by evaluating the axillary and internal mammary nodes.
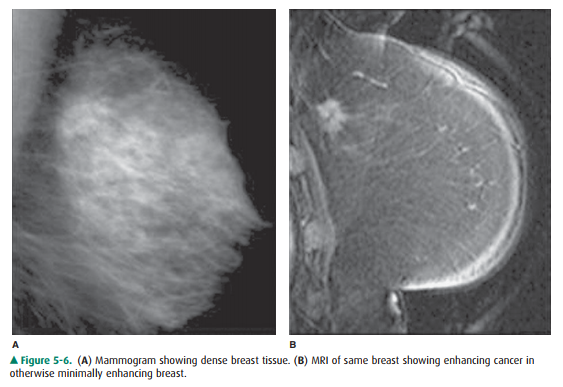
Figure 5-6 shows an enhancing cancerous tumor.
Although MRI is quite sensitive
(good for detecting dis-ease), it is relatively nonspecific. This is due to the
overlap-ping imaging characteristics of both benign and malignant processes.
Like cancer, some benign breast structures show enhancement, although usually
with a slower rate.
Because of the relatively low
specificity, screening with MRI is best used in patients with a higher
probability of disease. The 2007 American Cancer Society recommenda-tions
include annual MRI breast screening of patients with a lifetime risk of 20% or
greater.
Normal Structures
Tissues are differentiated by
their pattern of change on dif-ferent pulse sequences. The skin, nipple and
areola, mam-mary fat, breast parenchyma, and connective tissue are normally
seen, in addition to the anterior chest wall, in-cluding musculature, ribs and
their cartilaginous portions, and portions of internal organs. Small
calcifications are not visible, and small solid nodules may not be detected.
Cystic structures are well seen. Normal implants appear as cystic structures
with well-defined walls. Their location is deep to the breast parenchyma or
subpectoral, depending on the surgical technique that was used to place the
implants. In-ternal signal varies and depends on implant contents, either
silicone or saline.
Ductography
Ductography, or galactography, uses
mammographic imag-ing with contrast injection into the breast ducts. The
indica-tion for use is a profuse, spontaneous, nonmilky nipple discharge from a
single duct orifice. If these conditions are not present, the ductogram is
likely to be of little help. The purpose is to reveal the location of the
ductal system in-volved. The cause of the discharge is frequently not
identi-fied. Occasionally, an intraluminal abnormality is seen, but findings
have low specificity.
The patient lies in supine
position while the discharging duct is cannulated with a blunt-tipped needle or
catheter under visual inspection and with the aid of a magnifying glass. A
small amount of contrast material (usually not more than 1 mL) is injected
gently by hand into the duct. Several mammographic images are then made. The
procedure re-quires about 30 minutes and is not normally painful.
Normal Structures
Just deep to the opening of the
duct on the nipple, the duct ex-pands into the lactiferous sinus. After a few
millimeters, the duct narrows again and then branches as it enters the lobe
con-taining the glands drained by this ductal system. The normal caliber of the
duct and its branches is highly variable, but nor-mal duct walls should be
smooth, without truncation or abrupt narrowing. With high-pressure injection,
the lobules, as well as cystically dilated portions of ducts and lobules, may
opacify.
Image-Guided Needle Aspiration and Biopsy
The indications for needle
aspiration and biopsy of breast le-sions are varied and are variably
interpreted by radiologists and referring physicians. Two categories are
discussed here.
The first indication is
aspiration of cystic lesions to con-firm diagnosis, to relieve pain, or both.
Nonpalpable cysts re-quire either ultrasound or mammography to be seen. A fine
needle (20- to 25-gauge) usually suffices to extract the fluid. The cystic
fluid is not routinely sent for cytology unless it is bloody.
The second indication concerns
solid lesions. Needle biopsy is used in this case (1) to confirm benignity of a
lesion carrying a low suspicion of malignancy mammographically,to confirm
malignancy in a highly suspicious lesion prior to initiating further surgical
planning and treatment, and to evaluate any other relevant mammographic lesion
for which either follow-up imaging or surgical excision is a less desirable
option for further evaluation.
Guidance for needle biopsy can be
accomplished with stereotactic mammography, ultrasound, and MR. Imaging
modality for needle guidance is selected on the basis of lesion
characteristics, availability of technology, and personal pref-erence of the
radiologist. Ultrasound and mammography are the most commonly used techniques.
Large core biopsy (typically 14-,
11-, or 8-gauge) has been shown to be more accurate for nonpalpable lesions
than fine needle aspiration (20-gauge or smaller) and is often com-bined with
vacuum assistance to further increase tissue yield.
Mammographic guidance is most
easily and accurately performed with a stereotactic table unit. Lesions of only
a few millimeters can be successfully biopsied. With stereotac-tic tables, the
patient lies prone with the breast protruding through an opening in the table
surface. A needle is mechan-ically guided to the proper location in the breast
with com-puter assistance. The entire procedure requires 30 minutes to 1 hour.
Image-Guided Needle Localization
When a nonpalpable breast lesion
must be excised, imaging is used to guide placement of a needle into the
breast, with the needle tip traversing or flanking the lesion. Either
ultrasono-graphic or mammographic guidance can be used, and the choice again
depends on lesion characteristics and personal preference. Once the needle is in
the appropriate position, a hook wire is inserted through the needle to anchor
the device in place. This prevents migration during patient transport and
surgery. After needle placement, the patient is taken to the operating theater
for excision of the lesion by the surgeon.
Biopsy Specimen Radiography
When a lesion is excised from the
breast, a surgical specimen can be radiographed to document that the
mammographic abnormality was removed. This practice is routinely followed with
needle-localized lesions, but palpable lesions excised may also be radiographed
to confirm that the specimen con-tains an abnormality that may have been
present on the mammogram.
Related Topics