Chapter: Clinical Anesthesiology: Anesthetic Equipment & Monitors : Cardiovascular Monitoring
Pulmonary Artery Catheterization
PULMONARY ARTERY CATHETERIZATION
The pulmonary artery (PA) catheter (or
Swan-Ganz catheter) was introduced into routine prac-tice in operating rooms
and intensive care units in the 1970s. It quickly became common for patients
undergoing major surgery to be managed with PA catheterization. The catheter
provides measure-ments of both CO and PA occlusion pressures and was used to
guide hemodynamic therapy, especially when patients became unstable.
Determination of the PA occlusion or wedge pressure permitted (in the absence
of mitral stenosis) an estimation of the left ventricular end-diastolic
pressure (LVEDP), and, depending upon ventricular compliance, an estimate of
ventricular volume. Through its ability to perform measurements of CO, the
patient’s stroke volume (SV) was also determined.
CO = SV × HR
SV = CO/HR
Blood pressure = CO × systemic vascularresistance (SVR)
Consequently, hemodynamic monitoring
with the PA catheter attempted to discern why a patient was unstable so that
therapy could be directed at the underlying problem.
If the SVR is diminished, such as in
states of vasodilatory shock (sepsis), the SV may increase. Conversely, a
reduction in SV may be second-ary to poor cardiac performance or hypovolemia.
Determination of the “wedge” or pulmonary cap-illary occlusion pressure (PCOP)
by inflating the catheter balloon estimates the LVEDP. A decreased SV in the
setting of a low PCOP/ LVEDP indicates hypovolemia and the need for volume
adminis-tration. A “full” heart, reflected by a high PCOP/ LVEDP and low SV,
indicates the need for a positive inotropic drug. Conversely, a normal or
increased SV in the setting of hypotension could be treated with the
administration of vasoconstrictor drugs to restore SVR in a vasodilated
patient.
Although patients can present
concurrently with hypovolemia, sepsis, and heart failure, this basic treatment
approach and the use of thePA catheter to guide therapy became more or less
synonymous with perioperative intensive care and cardiac anesthesia. However,
numerous large obser-vational studies have shown that patients managed with PA
catheters had worse outcomes than similar patients who were managed without PA
catheters. Other studies seem to indicate that although PA catheter-guided
patient management may do no harm, it offers no specific benefits. Although the
PA catheter can be used to guide goal-directed hemodynamic therapy to ensure
organ per-fusion in shock states, other less invasive methods to determine
hemodynamic performance are available, including transpulmonary thermodilution
CO mea-surements and pulse contour analyses of the arterial pressure waveform.
Both methods permit calcula-tion of the SV as a guide for hemodynamic
manage-ment. Moreover, right atrial blood oxygen saturation, as opposed to
mixed venous saturation (normal is 75%), can be used as an alternative measure
to dis-cern tissue oxygen extraction and the adequacy of tissue oxygen
delivery.
Despite numerous reports of its
questionable utility and the increasing number of alternative methods to
determine hemodynamic parameters, the PA catheter is still employed
perioperatively more often in the United States than elsewhere. Although
echocardiography can readily inform hemodynamic decision-making through
imag-ing the heart to determine if it is full, compressed, contracting, or
empty echocardiography requires a trained individual to obtain and interpret
the images. Alternative less invasive hemodynamic monitors have gained
acceptance in Europe and may expand in the United States, further decreasing
the use of PA catheters.
Until other alternatives are available,
PA cath-eterization should be considered whenever cardiac index, preload,
volume status, or the degree of mixed venous blood oxygenation need to be
known. These measurements might prove particularly important in surgical
patients at high risk for hemodynamic instability (eg, those who recently
sustained myo-cardial infarction) or during surgical procedures associated with
an increased incidence of hemody-namic complications (eg, thoracic aortic
aneurysm repair).
Contraindications
Relative contraindications to pulmonary
artery catheterization include left bundle-branch block(because of the concern
about complete heart block) and conditions associated with a greatly increased
risk of arrhythmias, such as Wolff–Parkinson–White syndrome. A catheter with
pacing capability is better suited to these situations. A PA catheter may serve
as a nidus of infection in bacteremic patients or throm-bus formation in
patients prone to hypercoagulation.
Techniques & Complications
Although various PA catheters are
available, the most popular design integrates five lumens into a 7.5 FR
catheter, 110-cm long, with a polyvinylchlo-ride body (Figure 5–20). The lumens house
the

following: wiring to connect the
thermistor near the catheter tip to a thermodilution CO computer; an air
channel for inflation of the balloon; a proximal port 30 cm from the tip for
infusions, CO injections, and measurements of right atrial pressures; a
ventricular port at 20 cm for infusion of drugs; and a distal port for aspiration
of mixed venous blood samples and measurements of PA pressure.
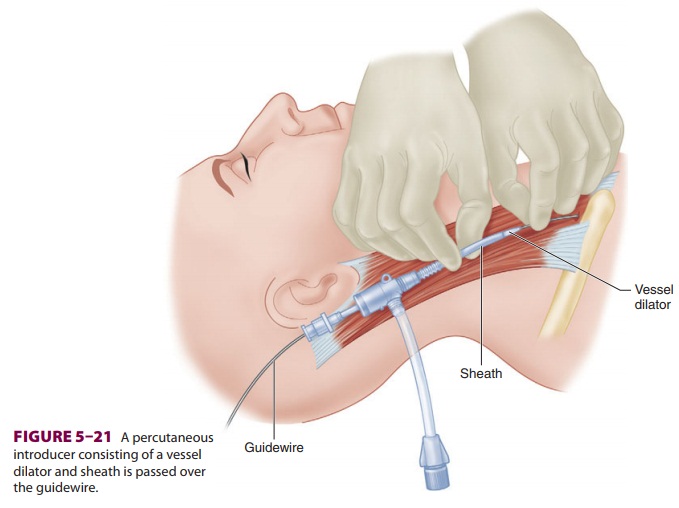
Insertion of a PA catheter requires
central venous access, which can be accomplished using Seldinger’s technique,
described above. Instead of a central venous catheter, a dilator and sheath are
threaded over the guidewire. The sheath’s lumen accommodates the PA catheter
after removal of the dilator and guidewire ( Figure 5–21).
Prior to insertion, the PA catheter is
checked by inflating and deflating its balloon and irrigating all three intravascular
lumens with saline. The distal port is connected to a transducer that is zeroed
to the patient’s midaxillary line.
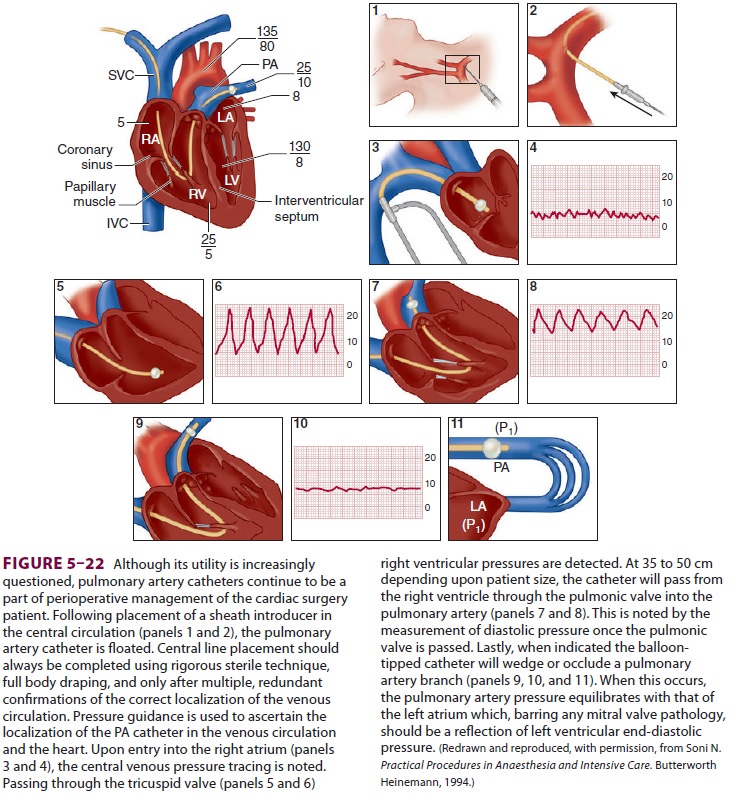
The PA catheter is advanced through the
intro-ducer and into the internal jugular vein. At approxi-mately 15 cm, the
distal tip should enter the right atrium, and a central venous tracing that
varies with respiration confirms an intrathoracic position. The balloon is then
inflated with air according to the manufacturer’s recommendations (usually 1.5
mL) to protect the endocardium from the catheter tip and to allow the right
ventricle’s CO to direct the catheter forward. The balloon is always deflated
during withdrawal. During the catheter’s advance-ment, the ECG should be
monitored for arrhyth-mias. Transient ectopy from irritation of the right
ventricle by the balloon and catheter tip is common and rarely requires
treatment. A sudden increase in the systolic
pressure on the distal tracing indi-cates a right ventricular location of the
catheter tip (Figure
5–22). Entry into the pulmonary artery nor-mally occurs by 35–45 cm
and is heralded by a sud-den increase in diastolic
pressure.
To prevent catheter knotting, the
balloon should be deflated and the catheter withdrawn if pressure changes do
not occur at the expected distances. In particularly difficult cases (low CO,
pulmo-nary hypertension, or congenital heart anomalies),flotation of the
catheter may be enhanced by having the patient inhale deeply; positioning the
patient in head-up, right lateral tilt position; injecting iced saline through
the proximal lumen to stiffen the cath-eter (which also increases the risk of
perforation); or administering a small dose of an inotropic agent to increase
CO. Occasionally, the insertion may require fluoroscopy or TEE for guidance.
After attaining a PA position, minimal
PA cath-eter advancement results in a pulmonary artery occlusion pressure
(PAOP) waveform. The PA trac-ing should reappear when the balloon is deflated.
Wedging before maximal balloon inflation signals an overwedged position, and
the catheter should be slightly withdrawn (with the balloon down, of course).
Because PA rupture may cause
mortality and can occur because of balloon overinflation, the frequency of
wedge readings should be minimized. PA pressure should be continuously
moni-tored to detect an overwedged position indicative of catheter migration.
Furthermore, if the catheter has a right ventricular port 20 cm from the tip,
distal migration can often be detected by a change in the pressure tracing that
indicates a pul-monary artery location.Correct catheter position can be
confirmed by a chest radiograph.
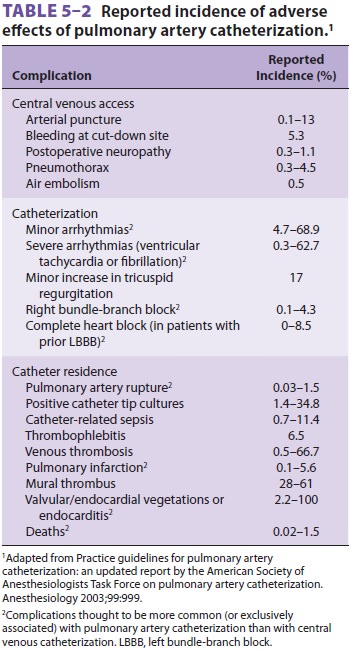
The numerous complications of PA
catheter-ization include all complications associated with central venous
cannulation plus bacteremia, endo-carditis, thrombogenesis, pulmonary
infarction, PA rupture, and hemorrhage (particularly in patients taking
anticoagulants, elderly or female patients, or patients with pulmonary
hypertension), catheter knotting, arrhythmias, conduction abnormalities, and
pulmonary valvular damage (Table 5–2). Even trace hemoptysis should not
be ignored, as it may herald PA rupture. If the latter is suspected, prompt
placement of a double-lumen tracheal tube may maintain adequate oxygenation by
the unaffected lung. The risk of complications increases with the duration of
catheterization, which usually should not exceed 72 hr.
Clinical Considerations
The introduction of PA catheters into
the operat-ing room revolutionized the intraoperative man-agement of critically
ill patients. PA catheters allow more precise estimation of left ventricular
preload than either CVP or physical examination, as well as the sampling of
mixed venous blood. Catheters with self-contained thermistors
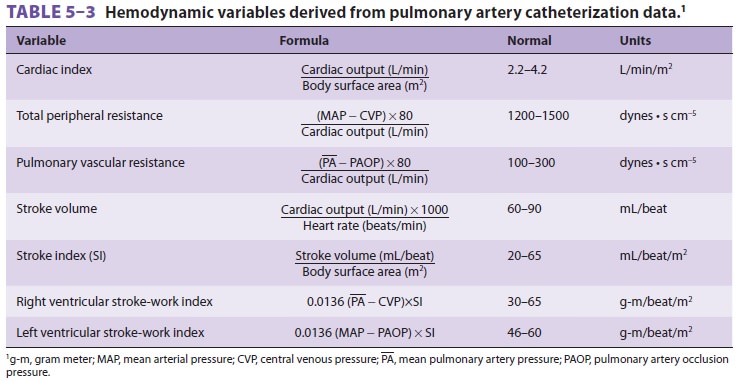
can be used to measure CO, from which a
multitude of hemodynamic values can be derived (Table 5–3). Some catheter
designs incorporate electrodes that allow intracavitary ECG recording and
pacing. Optional fiberoptic bundles allow con-tinuous measurement of the oxygen
saturation of mixed venous blood.
Starling demonstrated the relationship between left ventricular function and left ventricular end-diastolic muscle fiber length, which is usually proportionate to end-diastolic volume. If compli-ance is not abnormally decreased (eg, by myocar-dial ischemia, overload, ventricular hypertrophy, or pericardial tamponade), LVEDP should reflect fiber length. In the presence of a normal mitral valve, left atrial pressure approaches left ventricu-lar pressure during diastolic filling. The left atrium connects with the right side of the heart through the pulmonary vasculature. The distal lumen of a correctly wedged PA catheter is isolated from right-sided pressures by balloon inflation. Its distal open-ing is exposed only to capillary pressure, which—in the absence of high airway pressures or pulmonary vascular disease—equals left atrial pressure. In fact, aspiration through the distal port during balloon inflation samples arterialized blood. PAOP is an indirect measure of LVEDP which depending upon ventricular compliance approximates left ventricu-lar end diastolic volume.
Whereas central venous pressure may
reflect right ventricular function, a PA catheter may be indicated if either
ventricle is markedly depressed, causing disassociation of right- and
left-sided hemodynamics. CVP is poorly predictive of pul-monary capillary
pressures, especially in patients with abnormal left-ventricular function. Even
the PAOP does not always predict LVEDP. The relation-ship between left
ventricular end-diastolic volume (actual preload) and PAOP (estimated preload)
can become unreliable during conditions associated with changing left atrial or
ventricular compliance, mitral valve function, or pulmonary vein resistance.
These conditions are common immediately follow-ing major cardiac or vascular
surgery and in criti-cally ill patients who are on inotropic agents or in
septic shock.
Ultimately, the information provided by
the PA catheter is like that from any perioperative moni-tor dependent upon its
correct interpretation by the patient’s care givers. In this context, the PA
catheter is a tool to assist in goal-directed perioperative ther-apy. Given the
increasing number of less invasive methods now available to obtain similar
informa-tion, we suspect that PA catheterization will become mostly of historic
interest.
Related Topics