Chapter: Clinical Anesthesiology: Anesthetic Equipment & Monitors : Cardiovascular Monitoring
Electrocardiography
ELECTROCARDIOGRAPHY
Indications & Contraindications
All patients should have intraoperative
monitor-ing of their electrocardiogram (ECG). There are no contraindications.
Techniques & Complications
Lead selection determines the diagnostic
sensitivity of the ECG. ECG leads are positioned on the chest and extremities
to provide different perspectives of the electrical potentials generated by the
heart. At the end of diastole, the atria contract, which pro-vides the atrial
contribution to CO, generating the “P” wave. Following atrial contraction, the
ven-tricle is loaded awaiting systole. The QRS complex begins the electrical
activity of systole following the 120–200 msec atrioventricular (AV) nodal
delay. Depolarization of the ventricle proceeds from the AV node through the
interventricular system via the His–Purkinje fibers. The normal QRS lasts
approxi-mately 120 msec, which can be prolonged in patients with
cardiomyopathies and heart failure. The T wave represents repolarization as the
heart prepares to contract again. Prolongation of the QT interval sec-ondary to
electrolyte imbalances or drug effects can potentially lead to life-threatening
arrhythmias (les torsade de pointes).
The electrical axis of lead II is
approximately 60° from the right arm to the left leg, which is parallel to the
electrical axis of the atria, resulting in the larg-est P-wave voltages of any
surface lead. This orienta-tion enhances the diagnosis of arrhythmias and the
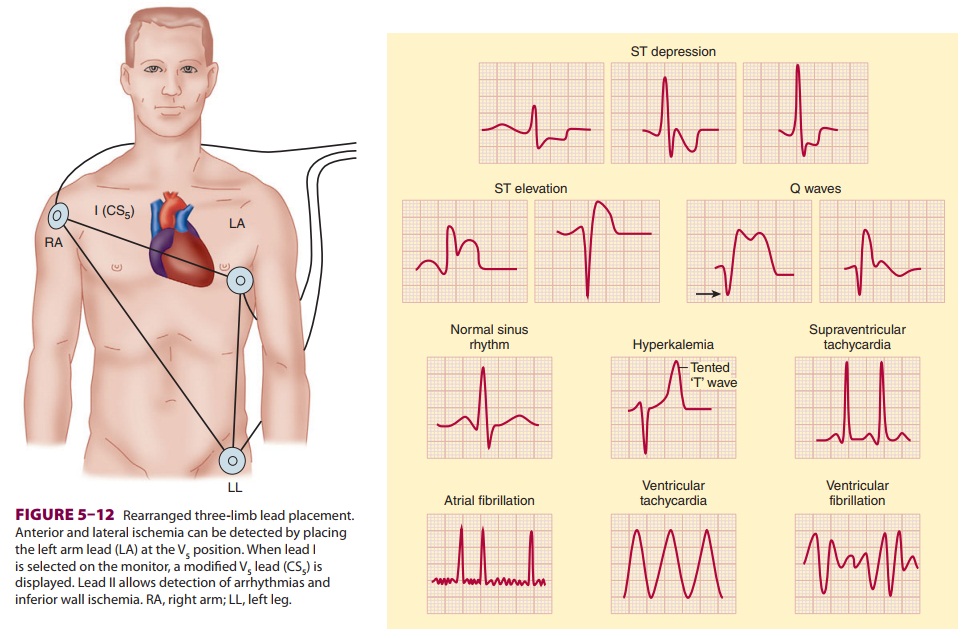
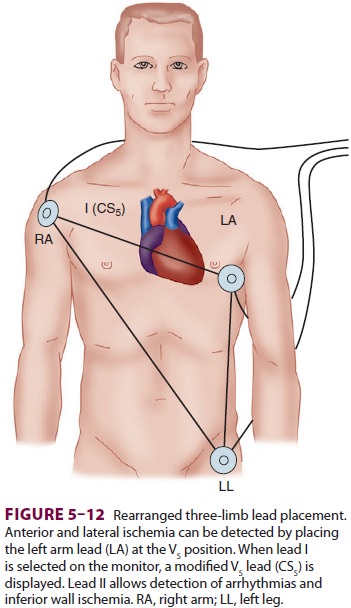
detection of inferior wall ischemia. Lead V 5
lies over the fifth intercostal space at the anterior axillary line; this
position is a good compromise for detecting anterior and lateral wall ischemia.
A true V5 lead is possible only on operating room
ECGs with at least five lead wires, but a modified V 5 can be monitored by rearranging the standard
three-limb lead place-ment (Figure 5–12). Ideally, because each lead
pro-vides unique information, leads II and V5
should be monitored simultaneously. If only a single-channel machine is
available, the preferred lead for monitor-ing depends on the location of any
prior infarction or ischemia. Esophageal leads are even better than lead II for
arrhythmia diagnosis, but have not yet gained general acceptance in the
operating room.
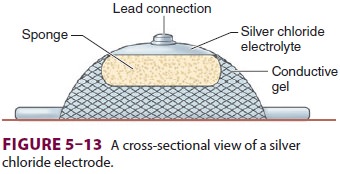
Electrodes are placed on the patient’s
body to monitor the ECG (Figure 5–13). Conductive gel lowers the skin’s
electrical resistance, which can be further decreased by cleansing the site
with alco-hol. Needle electrodes are used only if the disks are unsuitable (eg,
with an extensively burned patient).
Clinical Considerations
The ECG is a recording of the electrical
poten-tials generated by myocardial cells. Its routine use allows arrhythmias,
myocardial ischemia,
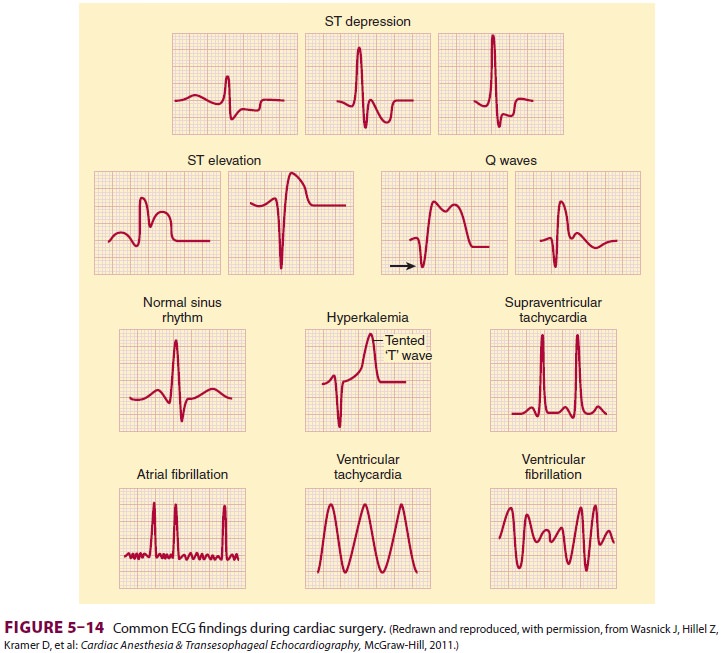
conduction abnormalities, pacemaker
malfunc-tion, and electrolyte disturbances to be detected (Figure 5–14). Because of the
small voltage poten-tials being measured, artifacts remain a major problem.
Patient or lead-wire movement, use of electrocautery, 60-cycle interference
from nearby alternating current devices, and faulty electrodes can simulate
arrhythmias. Monitoring filters incor-porated into the amplifier to reduce
“motion” arti-facts will lead to distortion of the ST segment and may impede
the diagnosis of ischemia. Digital readouts of the heart rate (HR) may be
misleading because of monitor misinterpretation of artifacts or large T
waves—often seen in pediatric patients—as QRS complexes.Depending on equipment
availability, a pre-induction rhythm strip can be printed or frozen on the
monitor’s screen to compare with intraop-erative tracings. To interpret
ST-segment changes properly, the ECG must be standardized so that a 1-mV signal
results in a deflection of 10 mm on a standard strip monitor. Newer units
continuously analyze ST segments for early detection of myo-cardial ischemia.
Automated ST-segment analysis increases the sensitivity of ischemia detection,
does not require additional physician skill or vigilance,and may help diagnose
intraoperative myocardial ischemia.
Commonly accepted criteria for
diagnos-ing myocardial ischemia require that the ECG be recorded in “diagnostic
mode” and include a flat or downsloping ST-segment depression exceed-ing 1 mm,
80 msec after the J point (the end of the QRS complex), particularly in
conjunction with T-wave inversion. ST-segment elevation with peaked T waves can
also represent ischemia. Wolff– Parkinson–White syndrome, bundle-branch blocks,
extrinsic pacemaker capture, and digoxin therapy may preclude the use of
ST-segment information. The audible beep associated with each QRS complex
should be loud enough to detect rate and rhythm changes when the anesthesiologist’s
visual attention is directed elsewhere. Some ECGs are capable of storing
aberrant QRS complexes for further analysis, and some can even interpret and
diagnose arrhyth-mias. The interference caused by electrocautery units,
however, has limited the usefulness of auto-mated arrhythmia analysis in the
operating room.
Related Topics