Chapter: Microbiology and Immunology: Virology, Virus: Orthomyxoviruses
Prevention and Control - Influenza Viruses Infections
Prevention and Control
This is based on the following:
a) Immunoprophylaxis by vaccines
b) Chemoprophylaxis
◗ Immunoprophylaxis by vaccines
Influenza A subtypes H1N1 and H3N2 are most common pre-vailing human influenza viruses. The trivalent vaccine used worldwide contains influenza A strains from H1N1 and H3N2, along with an influenza B strain. Influenza virus vaccines have been used for about 40 years to prevent influenza, primarily influenza A and B. Following types of vaccines are used:
Inactivated vaccines: Initially inactivated vaccines were used.The viruses for these vaccines are grown in chick embryos, inac-tivated by formalin, purified to some extent, and adjusted to a dosage known to elicit an antibody response in most individu-als. The vaccines contain the strains of types A and B viruses that are believed most likely to produce epidemics during the following winter.
The vaccine is administered parenterally. One or two doses are required, depending on the immune experience of the pop-ulation with related antigens. The vaccines are recommended especially for persons at high risk, especially those over 65 years of age and those with chronic cardiopulmonary diseases.
Local and systemic reactions to the vaccine are minor and occur in the first day or two after vaccination. In some persons, the vaccine may cause reactions allergic to egg proteins pres-ent in the vaccine. The killed vaccines do not induce formation of secretory antibodies in the secretory mucosa, although they elicit production of specific protective antibodies in the serum.
Live attenuated vaccines: Live attenuated vaccines are nowbeing developed as alternatives to inactivated vaccines. These vaccines induce production of specific secretory antibodies in respiratory mucosa. Earlier, the live vaccine used the viruses that were attenuated by repeated egg passage and was given by intranasal instillation. But these vaccines often failed to protect the children from clinical disease. Recently, temperature-sen-sitive (ts) mutant strains have been used in the live attenuated vaccine preparations. These avirulent mutants are able to grow at 32–34°C in the nasopharyngeal secretions, but not at 37°C in the lungs. These vaccines are useful to protect the children from clinical disease.
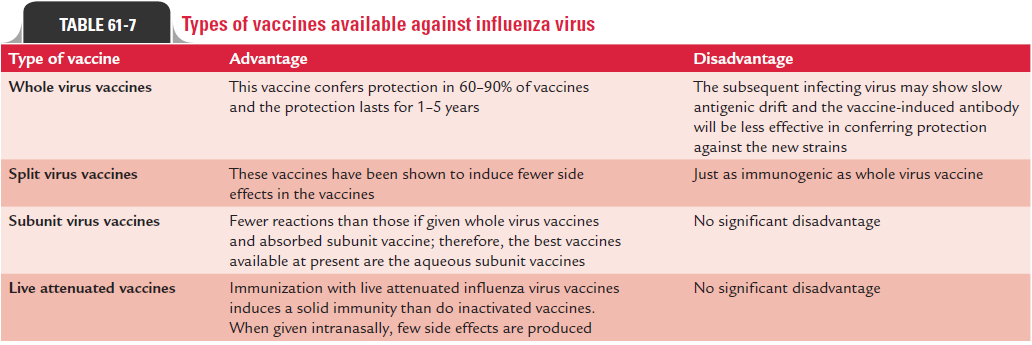
Table 61-7 summarizes vaccines available against influenza virus.
◗ Chemoprophylaxis
Chemoprophylaxis by amantadine and rimantadine hydrochlo-ride has been shown to be more successful. These two drugs effectively prevent infection and illness caused by type A, but not by type B viruses (because they lack M2 components). The persons with high risk can be protected by administering in a dosage of 100 mg/day. The drugs interfere with virus uncoat- ing and transport by blocking the transmembrane M2 ion channel. These antiviral agents prevent about 50% of infections and about 67% of illnesses under natural conditions. When administered for 10 days to household contacts of a person with influenza, these drugs protect up to 80% of the persons from illness. Side effects are greater for amantadine but are lim- ited to the central nervous system.
Related Topics