Chapter: Medical Surgical Nursing: Management of Patients With Infectious Diseases
Preventing Infection in the Hospital
PREVENTING INFECTION IN THE HOSPITAL
Nurses specializing in
infection control are responsible for agency-wide policy development and
program direction. Infection risk is significantly increased as patient care
equipment becomes more complex and as more devices that disrupt naturally
protective anatomic barriers are used. Staff nurses play an important role in
risk reduction by paying careful attention to hand hygiene, by ensuring careful
administration of prescribed antibiotics, and by following procedures to reduce
the risks associated with patient care devices.
Each year, an estimated 2 million patients in the United
States acquire nosocomial infections
while hospitalized. Approximately 240,000 additional residents of long-term
care facilities become infected each year. With the anticipated growth of the
elderly population, this number may increase to approximately 750,000 by 2005 (
Jarvis, 2001).
The CDC estimates that
approximately one third of all noso-comial infections could be prevented with
effective infection con-trol programs. An effective program includes the
following components: a program of surveillance for nosocomial infections and
vigorous control efforts, at least one infection control practi-tioner for
every 250 hospital beds, a trained hospital epidemiol-ogist, and feedback to
surgeons about individual surgical site infections. Unfortunately, many hospitals
have not introduced all four required aspects, and only an estimated 9% of
expected infections are prevented (Scheckler et al., 1998).
Specific Organisms With Nosocomial Infection Potential
CLOSTRIDIUM DIFFICILE
Clostridium difficile is a spore-forming bacterium with significantnosocomial
potential. Infection is usually preceded by antibiotics that disrupt normal
intestinal flora and allow the antibiotic-resistant C. difficile spores to proliferate within the intestine.
The organism causes
pathology by releasing toxins into the lumen of the bowel. In pseudomembranous
colitis, the most ex-treme form of C.
difficile infection, debris from the injured lumen of the bowel and from
white blood cells accumulate in the form of pseudomembranes or studded areas of
the colon. The destruc-tion of such a large anatomic area can produce profound
sepsis.
Because antibiotics are used so extensively in the
hospital setting, most hospitalized patients are at risk for infection with C. difficile. The nosocomial potential is
increased because thespore is relatively resistant to disinfectants and can be
spread on the hands of health care providers after contact with equipment that
has previously been contaminated with C.
difficile. Control is best achieved by intensifying cleaning, using Contact
Precautions for infected patients, and stressing glove use and hand hygiene for
all care workers.
METHICILLIN-RESISTANT
STAPHYLOCOCCUS
AUREUS
Methicillin-resistant S.
aureus (MRSA) is
a common nosoco-mial infection in hospitals and extended care facilities. MRSA
refers to S. aureus organisms that
are resistant to methicillin or its comparable pharmaceutical agents, oxacillin
and nafcillin. Because of the pathogenicity of S. aureus, there has been concern about antibiotic resistance since
the discovery of penicillin in the 1940s. Soon after penicillin was introduced,
S. aureus became all but universally
penicillin resistant. Fortunately, alternative therapies in the form of
cephalosporins and, more importantly, synthetic
penicillin solutions
such as methicillin were introduced. It was not until the late 1970s that S. aureus showed resistance to
methi-cillin. At that time, the prevalence of the organism was originally
linked epidemiologically to the IV/injecting drug use community. Since the late
1960s, however, MRSA has become increasingly more prevalent, and transmission
within hospitals and nursing homes has been well documented.
Linezolid and vancomycin
are the preferred alternative treat-ments for serious MRSA infection. However,
there is concern that MRSA will eventually also become resistant to these
med-ications because they are used so commonly. For the first time, in April
2002, a patient in Michigan was diagnosed with an S. aureus infection that was fully resistant to vancomycin (ie, vancomycin-resistant S. aureus [VRSA]). The CDC and other professionalorganizations
have focused preventive efforts against the threat of transmission of this
strain and the development of similar strains in other patients. The threat of
the growth of VRSA is considered a public health catastrophe because many
patients with S. aureus infections
are likely to have a poor outcome (CDC, 2002b).
Health care providers
often transmit MRSA to patients because S.
aureus easily colonizes skin. Because colonization is seldom rec-ognized,
the health care provider must assume that every
patient contact offers the possibility of MRSA exposure. Although there is no
evidence that MRSA is more virulent than other strains of staphylococci, the
colonized patient faces the likelihood of infec-tion with MRSA when invasive
procedures, such as intravenous therapy, respiratory therapy, or surgery, are
performed. The patient colonized with MRSA also serves as a reservoir of
resistant organ-isms to be transmitted to others. MRSA acquired in the hospital
may persist as normal flora in the patient in the future.
VANCOMYCIN-RESISTANT
ENTEROCOCCUS
Enterococcus is a gram-positive bacterium that is part of the nor-mal
flora of the gastrointestinal tract. It can produce significant disease when
allowed to infect blood, wounds, or urine. Entero-coccus
is the second most frequently isolated source of nosocomialinfection in the
United States.
Enterococcus has several traits that make it an ideal
nosocomialorganism. The host carries an abundance of the organism even in a
noninfected state; the organism is bile resistant and can with-stand harsh
anatomic sites, such as the intestine; Enterococcus
has the potential for resistance to many antibiotics, so that therapeutic
agents reducing local bacterial competition may leave it to repli-cate freely;
and the organism endures well on the hands of health care providers and on
environmental objects.
As a relatively
resistant organism at baseline, therapy for En-terococcus
has been essentially limited to penicillin formulations(eg, ampicillin) or
vancomycin in combination with an amino-glycoside (eg, gentamicin). In the
1980s, resistance to all of these agents was first reported. Between 1994 and
1999, the CDC recorded a more than 40% increase in the percentage of cases of vancomycin-resistant Enterococcus (VRE) infections in inten-sive care unit patients
(CDC, 2001c).
This rapidly growing
problem has serious implications. Be-cause many strains of VRE are resistant to
all other antimicrobial therapies, clinicians are left without effective
therapy for what was once seen as a relatively common infection. VRE infections
may serve as a reservoir of genes coded for vancomycin resistance that may be
transferred to the even more prevalent and virulent S. aureus. The first case of a patient infected with VRSA,
illustratesthis concern, as that patient was infected with VRE and VRSA.
The gene that commonly causes resistance in VRE was found
in both organisms, which strongly suggested genetic transfer be-tween species
(CDC, 2002b).
Preventing Nosocomial Bloodstream Infections (Bacteremia and Fungemia)
Reducing the risk of
nosocomial bloodstream infections requires preventive activities (in addition
to Standard and Transmission-Based Precautions, which are discussed later). If
a nosocomial bloodstream infection occurs, early diagnosis is important to
pre-vent complications, such as endocarditis and brain abscess. Mortality rates
may be as high as 25% for infection with some or-ganisms. The estimated cost
attributed to catheter-related blood-stream infections is $3,700 to $29,000 per
case (Mermel, 2000).
Bacteremia is
defined as laboratory-proven presence of bacte-ria in the bloodstream. Fungemia is a bloodstream infection
caused by a fungal organism. Any vascular access device (VAD) can serve as the
source for a bloodstream infection. Contamination can occur from the patient’s
own flora traversing the exterior of a catheter or by contamination of internal
tubing during manip-ulation. The intravenous fluid itself can become
contaminated and serve as a source of infection. Most hospitalized patients
receive VADs, and increasingly, long-term central catheters are used to provide
intravenous therapy to outpatients in a clinic or home setting. In all
instances, the nurse must use appropriate care to re-duce the risk of
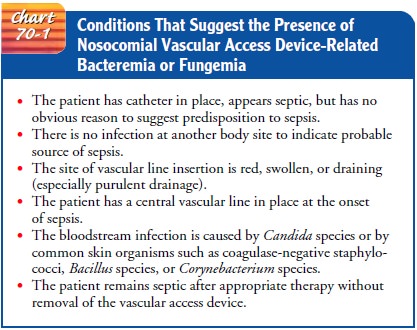
bacteremia and to be alert for signs of bacteremia. Chart 70-1 identifies
conditions that suggest the presence of noso-comial VAD-related bacteremia or
fungemia.
DISINFECTING SKIN
During the insertion of all VADs, there must be strict attention to aseptic technique. Those inserting VADs must vigorously wash their hands before insertion. Those inserting central catheters should use surgical technique, including sterile gloves, sterile gowns with long sleeves, masks, and a large drape over the patient. The preferred solution to disinfect the insertion site is chlorhexidine gluconate, which first became available as a skin preparation solution in the United States in 2001. Alternative so-lutions are povidone iodine or alcohol. Triple-antibiotic oint-ment should not be used on the insertion site because it has been shown to lead to increased colonization with Candida species (Mermel, 2000).
There is no apparent difference in risk or benefit when
com-paring the use of transparent polyurethane dressings or gauze dressings.
However, if blood is oozing from the catheter insertion site, a gauze dressing
should be used. The most important aspects for either material are that the
dressing should be applied using aseptic technique and that the dressing should
be sealed along its entire perimeter (Mermel, 2000).
USING GUIDE WIRES
Guide wires should not be used routinely when replacing
central venous catheters. However, they may be used if there is no evi-dence of
infection and insertion risk is unacceptably high, as when the patient has a
coagulopathy or is obese.
CHANGING INFUSION SETS, CAPS, AND SOLUTIONS
Infusion sets and
stopcock caps should be changed no more fre-quently than every 4 days, unless
an infusion set is used for the delivery of blood or lipid solutions. Infusion
sets and tubing for blood, blood products, or lipid emulsions should be changed
within 24 hours of initiating the infusion. Blood infusions should finish
within 4 hours of hanging the blood; lipid solutions should be completed within
24 hours of hanging. There are no guide-lines for the appropriate intervals for
the hang time of other so-lutions. Injection ports should be cleaned with 70%
alcohol or an iodophor before accessing the system (Mermel, 2000).
Nurses have an important
role in the prevention of blood-stream infections as they assess patients for
evidence of infection, make daily VAD site inspections, and monitor the
interval of line changes. Signs of sepsis in patients with indwelling vascular
lines should be promptly assessed and treated.
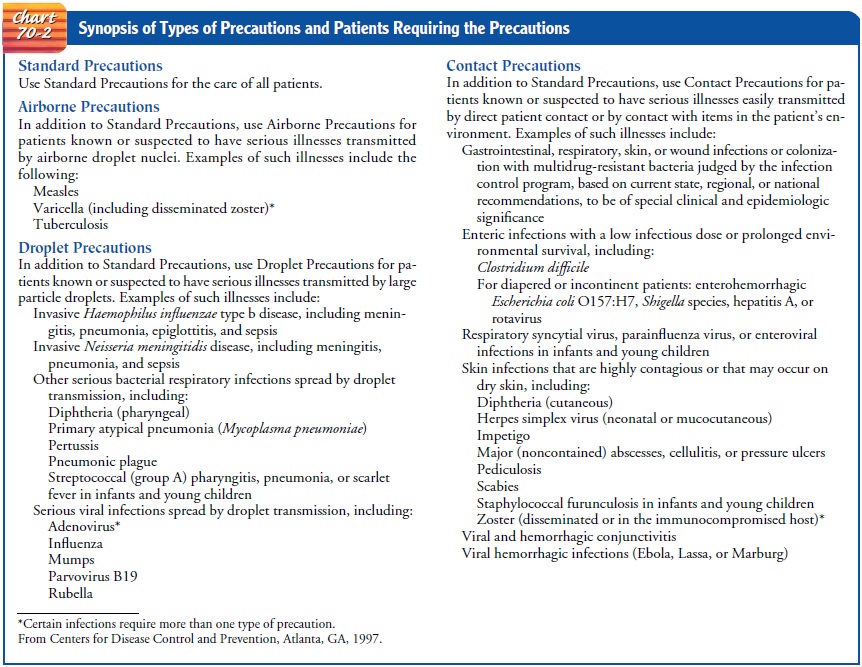
Isolation Precautions
Isolation precautions are guidelines created to prevent transmission of microorganisms in hospitals. In 1997, the Hospital Infection Control Practices
Advisory Committee (HICPAC), along with the CDC, implemented two tiers of
isolation precautions. The first tier, called Standard Precautions, was
designed for the care of all patients
in the hospital and is the primary strategy for preventing nosoco-mial
infections. The second tier, called Transmission-Based Pre-cautions, was
designed for care of patients with known or suspected infectious diseases
spread by airborne, droplet, or contact routes.
STANDARD PRECAUTIONS
The tenets of Standard Precautions are that all
patients are col-onized or infected with microorganisms, whether or not there
are signs or symptoms, and that a uniform level of caution should be used in
the care of all patients. The elements of Standard Pre-cautions include hand
hygiene, use of gloves and other barriers (eg, mask, eye protection, face
shield, gown), handling of patient care equipment and linen, environmental
control, prevention of injury from sharps devices, and patient placement. Hand
hygiene, glove use, needlestick prevention, and avoidance of splash or spray of
body fluids are discussed in the following sections.
Hand Hygiene.
The most frequent cause of infection outbreaksin
health care institutions is transmission by the hands of health care workers.
Hands should be washed or decontaminated fre-quently during patient care. Table
70-3 describes the recom-mended situations for hand hygiene.

When hands are visibly
dirty or contaminated with biologic material from patient care, hands should be
washed with soap and water. In intensive care units and other locations in
which viru-lent or resistant organisms are likely to be present, antimicrobial
agents (eg, chlorhexidine gluconate, iodophors, chloroxylenol, triclosan) may
be used. Effective hand washing requires at least 15 seconds of vigorous scrubbing with special attention to the
areaaround nail beds and between fingers, where there is high bacte-rial
burden. Hands should be thoroughly rinsed after this washing.
If hands are not visibly
soiled, health care providers are strongly encouraged to use alcohol-based,
waterless antiseptic agents for routine hand decontamination. These solutions
are superior to soap or antimicrobial handwashing agents in their speed of
action and effectiveness against bacteria and viruses. Because they are
formulated with emollients, they are usually better tolerated than other
agents, and because they can be used without sinks and towels, health care
workers have been found to be more compli-ant with their use. Nurses working in
home health care or other settings where they are relatively mobile should
carry pocket-sized containers of alcohol-based solutions (Zaragoza et al.,
1999).
Normal skin flora
usually consist of coagulase-negative staphy-lococci or diphtheroids. In the
health care setting, employees may temporarily carry bacteria (ie, transient flora) such as S. aureus,Pseudomonas aeruginosa, and
other organisms with strong patho-genic potential. Generally, transient flora
are superficially at-tached and are shed with hand hygiene and skin
regeneration.
Hand washing or disinfection reduces the amount of benign
normal flora and transient bacteria and decreases the risk of trans-fer to
other patients. All health care settings should have programs to evaluate
compliance with hand disinfection by all who care for patients.
Nurses should not wear
artificial fingernails or extenders when providing patient care. These items
have been epidemiologically linked to several significant outbreaks of
infections. Natural nails should be kept less than 0.25-inch (0.6-cm) long, and
nail polish should be removed when chipped, because it can support in-creased
bacterial growth (CDC, 2002a).
Glove Use.
Gloves provide an effective barrier for hands from themicroflora associated
with patient care. Gloves should be worn when a health care worker has contact
with any patient’s secretions or excretions and must be discarded after each
patient care con-tact. Because hospital organisms colonizing health care
workers’ hands can proliferate in the warm, moist environment provided by
gloves, hands must be thoroughly washed with soap after gloves are removed. As
patient advocates, nurses have an important role in promoting hand washing and
glove use by other hospital work-ers, such as laboratory personnel,
technicians, and others who have contact with patients.
Latex gloves are often
preferred over vinyl gloves because of greater comfort and fit and because some
studies indicate that they afford greater protection from exposure. Their
increased use in recent years, however, has been accompanied by increased
re-ports of allergic reactions to latex among health care workers. Re-actions
range from local skin irritation to more severe reactions, including
generalized dermatitis, conjunctivitis, asthma, angio-edema, and anaphylaxis.
The nurse who
experiences irritation or allergic reaction asso-ciated with exposure to latex
should report symptoms to an occu-pational health specialist or private
physician. Suggested methods for reducing the incidence of such reactions
include use of vinyl gloves, powder-free gloves, or “low-protein” latex gloves.
Needlestick Prevention.
The most important
aspect of reducingthe risk of bloodborne infection is avoidance of percutaneous
in-jury. Extreme care is essential in all situations in which needles,
scalpels, and other sharp objects are handled. Used needles should not be
recapped. Instead, they are placed directly into puncture-resistant containers
in the vicinity of their use. If a situation dictates that a needle must be recapped,
the nurse must use a me-chanical device to hold the cap or use a one-handed
approach to decrease the likelihood of skin puncture. Since 2001, OSHA has
required nurses to use needleless devices and other instruments designed to
prevent injury from sharps when appropriate (Occu-pational Safety and Health
Administration [OSHA], 2001).
Avoidance of Spray and Splash Exposure.
When
the health careprovider is involved in an activity in which body fluids may be
sprayed or splashed, appropriate barriers must be used. If a splash to the face
may occur, goggles and facemask are warranted. If the health care worker is
handling material that may soil clothing or is involved in a procedure in which
clothing may be splashed with biologic material, a cover gown should be worn.
TRANSMISSION-BASED PRECAUTIONS
Some microbes are so
contagious or epidemiologically significant that precautions in addition to the
Standard Precautions should be used when such organisms are recognized. The CDC
recom-mends a second tier of precautions, called Transmission-Based
Precautions. The additional safety measures are called Airborne, Droplet, and
Contact Precautions (Garner, 1996).
Airborne Precautions are required for patients with presumedor proven
pulmonary TB or chickenpox. Airborne Precautions are also advised if, as a
victim of bioterrorism, a patient is sus-pected of having smallpox. When
hospitalized, patients should be put in rooms with negative air pressure; the
door should remain closed, and health care providers should wear an N-95
respirator (ie, protective mask) at all times while in the patient’s room.
Droplet Precautions are used for organisms that can be trans-mitted by close,
face-to-face contact, such as influenza or menin-gococcal meningitis. While
taking care of a patient requiring
Droplet Precautions, the nurse should wear a facemask,
but be-cause the risk of transmission is limited to close contact, the door may
remain open. The CDC advises that negative-pressure rooms should be used in
hospitals if available.
Contact Precautions are used for organisms that are spread byskin-to-skin
contact, such as antibiotic-resistant organisms or C. difficile. Contact Precautions are designed to emphasize
cau-tious technique for organisms that have serious epidemiologic consequences
or those easily transmitted by contact between health care worker and patient.
The principles of transmission control used in the Standard Precautions are
accentuated. When possible, the patient requiring contact isolation is placed
in a pri-vate room to facilitate hand hygiene and protection of garments from
environmental contamination. Masks are not needed, and doors do not need to be
closed (Chart 70-2).
Related Topics