Chapter: Modern Analytical Chemistry: Basic Tools of Analytical Chemistry
Preparing Solutions of Analytical Chemistry
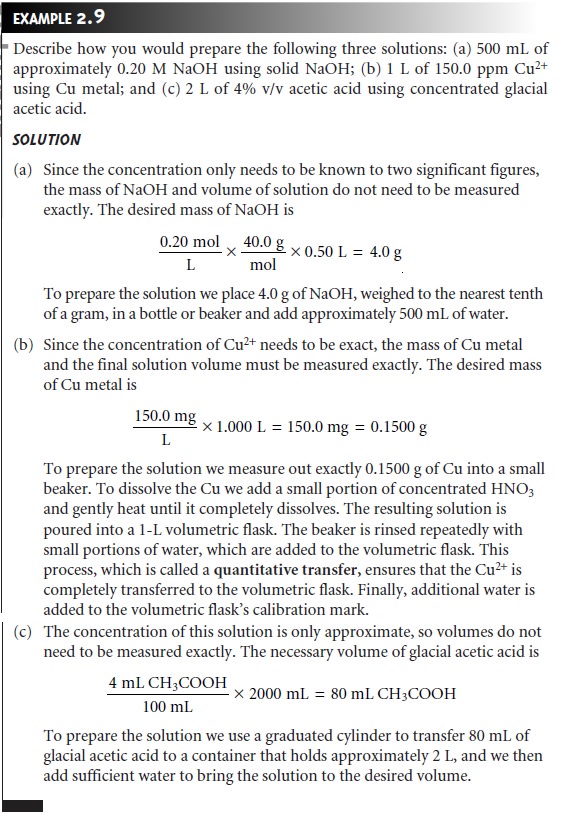
Preparing Solutions
Preparing a solution
of known concentration is perhaps the most common activity
in any analytical lab. The method for measuring out the solute
and solvent depend on the desired concentration units, and how exact the solution’s concentration needs to be known. Pipets
and volumetric flasks
are used when
a solution’s concen- tration must be exact;
graduated cylinders, beakers, and reagent bottles
suffice when concentrations need only be approximate. Two methods for preparing solutions are described in this section.
Preparing Stock Solutions
A
stock solution is prepared by weighing out
an appropriate portion
of a pure solid or by measuring
out an appropriate volume of a pure liquid and diluting
to a known volume.
Exactly how this is done depends on the required
concentration units. For example,
to prepare a solution with a desired
molarity you would
weigh out an appropriate mass of the reagent, dissolve
it in a portion of solvent, and bring
to the desired volume. To prepare a solution where
the solute’s concentration is given as a volume percent,
you would measure
out an appropriate volume of solute
and add sufficient solvent to obtain the
desired total volume.
Preparing Solutions by Dilution
Solutions with small
concentrations are often
prepared by diluting
a more concen- trated stock solution.
A known volume of the stock solution
is transferred to a new container and brought to a new volume. Since
the total amount
of solute is the
same before and after dilution, we
know that
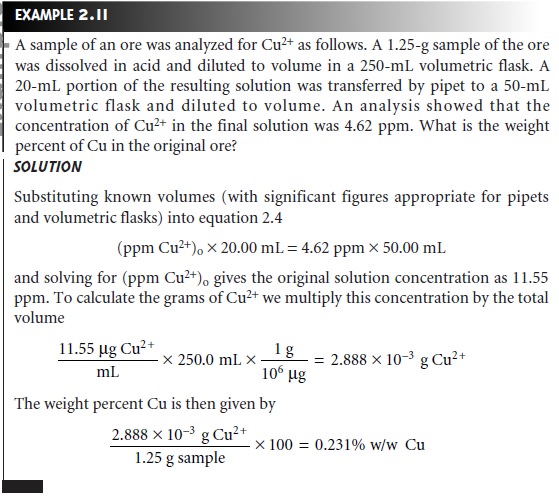
Co x Vo = Cd x Vd
………………….2.4
where Co is the concentration of the stock
solution, Vo is the volume
of the stock solution being diluted,
Cd is the concentration of the dilute
solution, and Vd is the
volume of the dilute solution. Again, the type of glassware used to measure
Vo and Vd depends
on how exact the solution’s concentration must be known.

As
shown in the following example,
equation 2.4 also can be used to calculate a solution’s original concentration using
its known concentration after dilution.
Related Topics