Chapter: Modern Analytical Chemistry: Basic Tools of Analytical Chemistry
Numbers in Analytical Chemistry
Numbers in Analytical Chemistry
Analytical chemistry is inherently a quantitative science.
Whether determining the concentration of a species
in a solution, evaluating an equilibrium constant, mea- suring a reaction
rate, or drawing
a correlation between
a compound’s structure and its reactivity, analytical
chemists make measurements and perform calculations. In this section we briefly review several important
topics involving the use of num-
bers in analytical chemistry.
Fundamental Units of Measure
Imagine that you find the following instructions in a laboratory procedure: “Trans- fer 1.5 of your sample to a 100 volumetric flask,
and dilute to volume.” How do you do
this? Clearly these
instructions are incomplete since the units
of measurement are not stated. Compare
this with a complete instruction: “Transfer 1.5 g of your sample to a 100-mL
volumetric flask, and dilute to volume.” This is an instruction
that you can easily follow.
Measurements usually consist
of a unit and a number expressing the quantity of that unit. Unfortunately, many different units
may be used to express
the same physical measurement. For example, the mass of a sample weighing 1.5 g also may
be expressed as 0.0033 lb or 0.053 oz. For consistency, and to avoid confusion, sci- entists use a common set of fundamental units, several of which are listed in Table
2.1. These units are called SI units after
the Système International d’Unités. Other measurements are
defined using these
fundamental SI units.
For example, we mea-
sure the quantity of heat produced during
a chemical reaction
in joules, (J), where
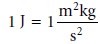
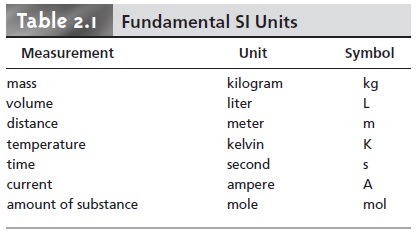
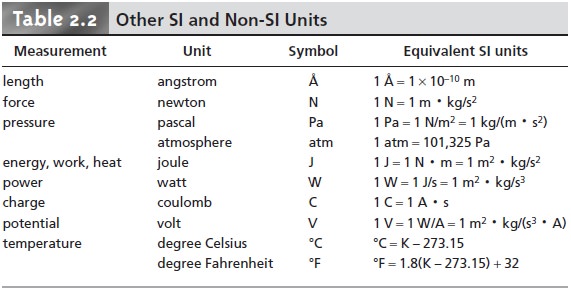
Table 2.2 provides
a list of other important derived SI units,
as well as a few com-
monly used non-SI units.
Chemists frequently work with
measurements that are
very large or very small. A mole, for example, contains
602,213,670,000,000,000,000,000 particles, and some analytical techniques can detect as little as 0.000000000000001 g of a compound.
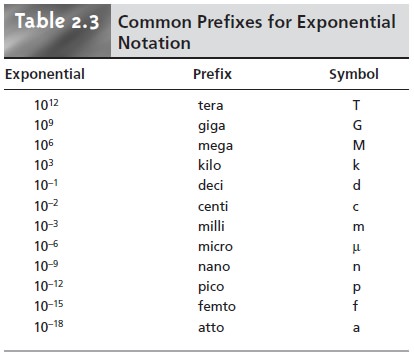
For simplicity, we express these
measurements using scientific notation; thus, a mole contains
6.0221367 x 1023 particles, and the stated
mass is 1 x 10–15 g. Some- times it is preferable to express measurements without the exponential term, replac- ing it with a prefix. A mass of 1 x 10–15 g
is the same as 1 femtogram. Table
2.3 lists other common prefixes.
Significant Figures
Recording a measurement provides information about
both its magnitude and un- certainty. For example, if we weigh
a sample on a balance
and record its mass as 1.2637 g, we assume
that all digits,
except the last,
are known exactly.
We assume that the
last digit has
an uncertainty of at least
±1, giving an absolute uncertainty of at least ±0.0001
g, or a relative uncertainty of at least
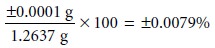
Significant figures are a reflection of a measurement’s uncertainty. The num- ber of significant figures
is equal to the number
of digits in the measurement, with the exception that
a zero (0)
used to fix
the location of a decimal
point is not
con- sidered significant. This definition can be ambiguous. For example, how many sig- nificant figures are in the number 100? If measured to the nearest
hundred, then there is one significant figure. If measured
to the nearest ten, however,
then two significant figures
are included. To avoid ambiguity we use scientific notation. Thus, 1 x 102 has one significant figure,
whereas 1.0 x 102 has two significant figures.
For
measurements using logarithms, such as pH,
the number of significant
figures is equal to the number of digits to the right of the decimal, including
all zeros. Digits to the left of the decimal are not included
as significant figures
since they only indicate
the power of 10. A pH of 2.45, therefore, contains two signifi- cant figures.
Exact numbers, such as the stoichiometric coefficients in a chemical formula
or reaction, and unit
conversion factors, have
an infinite number
of significant figures. A mole of CaCl2, for example, contains exactly two moles
of chloride and
one mole of calcium.
In the equality
1000 mL = 1 L
both numbers have an infinite number of significant figures.
Recording a measurement to the correct
number of significant figures is im- portant
because it tells
others about how precisely you made your measurement. For example, suppose you weigh an object on a balance capable of measuring mass to the nearest
±0.1 mg, but record its mass as 1.762 g instead of 1.7620 g. By
failing to record
the trailing zero,
which is a significant figure,
you suggest to others that the mass was determined using a balance
capable of weighing
to only the nearest
±1 mg. Similarly, a buret with scale markings every 0.1 mL can be read
to the nearest
±0.01 mL. The
digit in the
hundredth’s place is the least
sig- nificant figure since
we must estimate
its value. Reporting a volume of 12.241 mL
implies that your buret’s scale
is more precise
than it actually
is, with divi- sions every 0.01 mL.
Significant figures are
also important because
they guide us in reporting the re- sult of an analysis.
When using a measurement in a calculation, the result of that
calculation can never
be more certain
than that measurement’s uncertainty. Simply
put, the result of an analysis can never be more certain
than the least certain mea- surement included in the analysis.
As a general
rule, mathematical operations involving addition and subtraction
are carried out to the
last digit that
is significant for
all numbers included in the cal- culation. Thus, the sum
of 135.621, 0.33,
and 21.2163 is 157.17 since
the last digit that is significant for all three
numbers is in the hundredth’s place.
135.621 + 0.33 + 21.2163
= 157.1673 = 157.17
When multiplying and dividing, the general rule is that the answer
contains the same number
of significant figures
as that number
in the calculation having the fewest significant
figures. Thus,
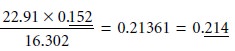
It
is important to remember, however,
that these rules
are generalizations. What is conserved is not the number of significant figures,
but absolute uncertainty when adding or subtracting, and relative uncertainty when multiplying or dividing.
For example, the following calculation reports the answer
to the correct number of significant figures, even though
it violates the
general rules outlined earlier.
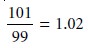
Since the relative uncertainty in both measurements is roughly 1% (101 ±1, 99 ±1), the
relative uncertainty in the final answer also must be roughly 1%. Reporting the answer to only two significant figures
(1.0), as required
by the general rules, implies a relative uncertainty of 10%. The correct answer,
with three significant figures, yields the expected
relative uncertainty.
Finally, to avoid “round-off ” errors in calculations, it is a good idea
to retain at least one extra significant figure throughout the
calculation. Better yet,
invest in a good scientific calculator that allows you to perform lengthy
calculations without recording intermediate values. When the calculation is complete, the final answer can be rounded to the correct
number of significant figures
using the following simple rules.
·
Retain the least significant figure if it and the
digits that follow
are less than halfway to the next
higher digit; thus, rounding 12.442 to the
nearest tenth gives 12.4 since 0.442
is less than halfway between
0.400 and 0.500.
·
Increase the least significant figure by 1 if it and the digits that follow are more
than halfway to the next higher digit;
thus, rounding 12.476
to the nearest tenth gives 12.5 since 0.476
is more than halfway between
0.400 and 0.500.
·
If
the least significant figure and the
digits that follow
are exactly halfway
to the next higher
digit, then round
the least significant figure to the
nearest even number; thus,
rounding 12.450 to the nearest
tenth gives 12.4,
but rounding 12.550 to the nearest tenth
gives 12.6. Rounding
in this manner
prevents us from introducing a bias by always rounding up or down
Related Topics