Chapter: Organic Chemistry: Amines and nitriles
Preparation of amines
PREPARATION OF AMINES
Key Notes
Reduction
Nitriles
can be reduced to primary amines with lithium aluminum hydride (LiAlH4).
Primary, secondary, and tertiary amides can be reduced with LiAlH4
to primary, secondary, and tertiary amines respectively.
Substitution with NH2
Nucleophilic
substitution of an alkyl halide with an azide ion gives an alkyl azide which
can then be reduced with LiAlH4 to give a primary amine.
Alternatively, nucleophilic substitution of an alkyl halide with a phthalim-ide
ion is carried out and the N-alkylated
phthalimide is then hydrolyzed to the primary amine. Reductive amination of an
aldehyde with ammonia is a third method of introducing an NH2 group.
A fourth possible method is to react an alkyl halide with ammonia, but this is
less satisfactory since over-alkylation is possible.
Alkylation of alkylamines
Primary
and secondary alkylamines can be alkylated to secondary and ter-tiary
alkylamines, respectively, by reaction with an alkyl halide. Primary
alkylamines can also be synthesized if ammonia is used instead of an
alkyl-amine. However, these reactions are difficult to control and
over-alkylation is common. Reductive amination is a more controlled method of
adding an extra alkyl group to an amine, where the amine (or ammonia) is
treated with an aldehyde or a ketone in the presence of a reducing agent (sodium
cyanoborohydride). Alternatively, primary and secondary amines can be acylated
with an acid chloride or acid anhydride and then reduced with LiAlH4
to give a secondary and tertiary amine, respectively.
Rearrangements
The
Hofmann and Curtius rearrangements are used to convert a carboxylic acid
derivative to a primary amine with the loss of a carbon unit – the original
carbonyl group. In both cases the rearrangement reaction involves the alkyl
group being transferred from the carbonyl group to the nitrogen atom to form an
isocyanate intermediate. Hydrolysis then results in loss of the original
carbonyl group. The Hofmann rearrangement involves the treatment of a primary
amide with bromine under basic conditions. The Curtius rearrangement involves
heating an acyl azide.
Arylamines
Amino
groups cannot be directly introduced to an aromatic ring. However, nitro groups
can be added directly by electrophilic substitution, then reduced to the amine.
Once the amine is present, reactions such as alkyla-tion, acylation, or
reductive amination can be carried out as described for alkylamines.
Reduction
Nitriles and amides can be reduced to
alkylamines using lithium aluminum hydride. In the case of a nitrile, a primary
amine is the only possible product. Primary, secondary, and tertiary amines can
be prepared from primary, secondary, and tertiary amides, respectively.
Substitution
with NH2
Primary alkyl halides and some secondary alkyl halides
can undergo SN2 nucleophilic substitution with an azide ion (N3
- ) to give an alkyl azide.
The overall reaction is equivalent to replacing
the halogen atom of the alkyl halide with an NH2 unit. Another
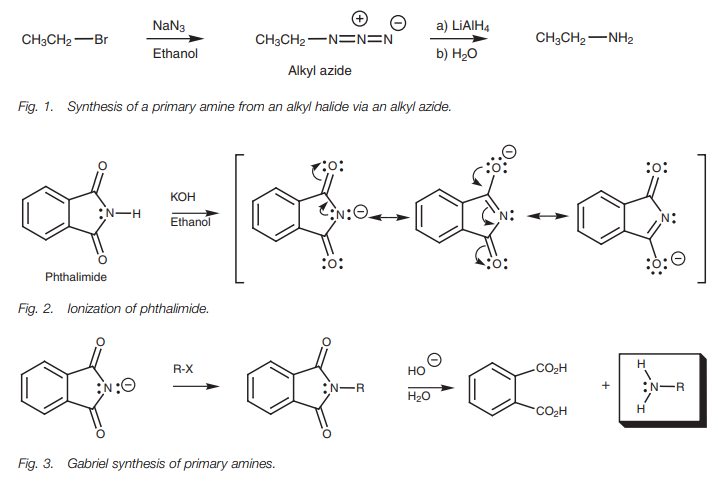
method of achieving the same result is the Gabriel
synthesis of amines. This involves treating phthalimide with KOH toabstract
the N–H proton (Fig. 2). The N–H
proton of phthalimide is more acidic (pKa
9) than the N–H proton of an amide since the anion formed can be stabilized by
resonance with both neighboring carbonyl groups. The phthalimide ion can then
be alkylated by treating it with an alkyl halide in a nucleophilic
substitution. Subsequent hydrolysis
releases a primary amine (Fig. 3).
A third possible method is to react an alkyl halide with ammonia, but this is less satisfactory since over-alkylation is possible (see below). The reaction of an alde-hyde with ammonia by reductive amination is a fourth method of obtaining primary amines (see below).
Alkylation of alkylamines
It is possible to convert primary and secondary
amines to secondary and tertiary amines respectively, by alkylation with alkyl
halides by the SN2 reaction. However, over-alkylation can be a problem and
better methods of amine syn-thesis are available.
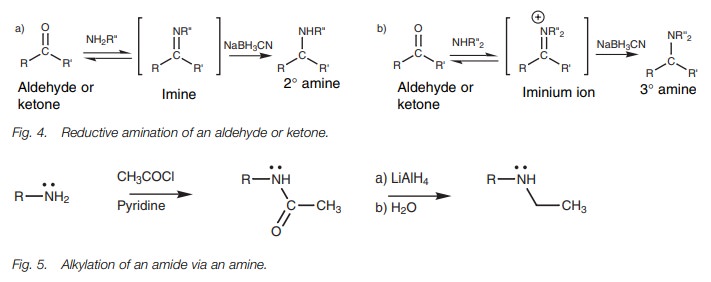
Reductive amination is a more controlled method
of adding an extra alkyl group to an alkylamine (Fig. 4). Primary and secondary alkylamines can be treated with a
ketone or an aldehyde in the presence of a reducing agent called sodium
cyanoborohydride. The alkylamine reacts with the carbonyl compound by
nucleo-philic addition followed by elimination to give an imine or an iminium
ion which is immediately reduced by sodium cyanoborohydride to give the final
amine. Overall, this is the equivalent of adding one extra alkyl group to the
amine. Therefore, primary amines are converted to secondary amines and
secondary amines are converted to tertiary amine. The reaction is also suitable
for the synthesis of primary amines if ammonia is used instead of an
alkylamine. The reaction goes through an imine intermediate if ammonia or a
primary amine is used (Fig. 4a). When
a secondary amine is used, an iminium ion intermediate is involved (Fig. 4b).
An alternative way of alkylating an amine is to acylate the amine to give an amide, and then carry out a reduction with LiAlH4. Although two steps are involved, there is no risk of over-alkylation since acylation can only occur once.
Rearrangements
There are two rearrangement reactions which can
be used to convert carboxylic acid derivatives into primary amines where the
carbon chain in the product has been shortened by one carbon unit (Fig. 6). These are known as the Hofmann
and the Curtius rearrangements. The Hofmann rearrangement involves the
treatment of a primary amide with bromine under basic conditions, while the
Curtius rearrangement involves heating an acyl azide. The end result is the
same – a primary amine with loss of the original carbonyl group.
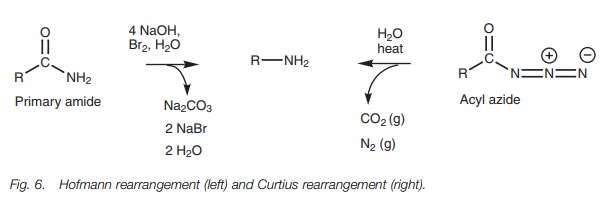
In both reactions, the alkyl group (R) is
transferred from the carbonyl group to the nitrogen to form an intermediate
isocyanate (O=C=N–R). This is then hydrolyzed by water to form carbon dioxide
and the primary amine. The Curtius rearrangement has the added advantage that
nitrogen is lost as a gas which helps to drive the reaction to completion.
Arylamines
The direct introduction of an amino group to an aromatic ring is not possible. However, nitro groups can be added directly by electrophilic substitution and then reduced to the amine. The reduction is carried out under acidic conditions resulting in an arylaminium ion as product. The free base can be isolated by basifying the solution with sodium hydroxide to precipitate the arylamine.
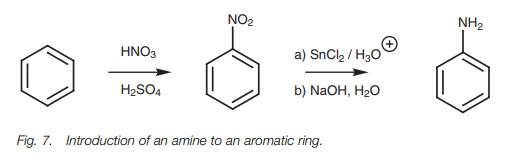
Once an amino group has been introduced to an
aromatic ring, it can be alkyl-ated with an alkyl halide, acylated with an acid
chloride or converted to a higher amine by reductive animation as described for
an alkylamine.
Related Topics