Chapter: Organic Chemistry: Amines and nitriles
Properties of amines
PROPERTIES OF AMINES
Key Notes
Structure
Amines
consist of an sp3 hybridized nitrogen linked to three substituents
by σ bonds. The functional group is pyramidal in shape with bond angles of
approximately 109°. If
the substituents are
alkyl groups, the
amine is aliphatic or an
alkylamine. If one or more of the substituents is aromatic, the amine is
aromatic or an arylamine. If the amine has only one alkyl or aryl substituent,
it is defined as primary. If there are two such substituents, the amine is
secondary, and if there are three such groups, the amine is tertiary.
Pyramidal inversion
Amines
can be chiral if they have three different substituents. However, it is not
possible to separate enantiomers since they can easily interconvert by
pyramidal inversion. The process involves a planar intermediate where the nitrogen
has changed from sp3
hybridization to sp2
hybridization and the lone pair of electrons are in a p orbital.
Pyramidal inversion is not possible for chiral quaternary ammonium salts and
enantiomers of these structures can be separated.
Physical Properties
Amines
are polar compounds with higher boiling points than comparable alkanes. They
have similar water solubilities to alcohols due to hydrogen bonding, and low
molecular weight amines are completely miscible with water. Low molecular
weight amines have an offensive fishy smell.
Basicity
Amines
are weak bases which are in equilibrium with their ammonium ion in aqueous
solution. The basic strength of an amine is indicated by its pKb
value. There are two main effects on basic strength. Alkyl groups have an
inductive effect which stabilizes the ammonium ion and results in increased
basicity. Solvation of the ammonium ion by water stabilizes the ion and
increases basicity. The more hydrogen bonds which are possible between the
ammonium ion and water, the greater the stability and the greater the basicity.
The alkyl inductive effect is greates t for ammonium ions formed from tertiary
amines, whereas the solvation effect is greatest for ammoniumions formed
from primary amines.
In general, primary
and secondary amines are
stronger bases than
tertiary amines. Aromatic
amines are weaker bases than aliphatic
amines since nitrogen’s lone pair of electrons interacts with the π system of
the aromatic ring, and is less likely to form a bond to aproton. Aromatic
substituents affect basicity. Activating sub- stituents increase electron
density in the aromatic ring which helps to stabi- lize the ammonium ion and
increase basic strength. Deactivating groups have the opposite effect.
Substituents capable of interacting with the aro-matic ring by resonance have a
greater effect on basicity if they are at theortho or para positions.
Reactivity
Amines react as nucleophiles or bases since they have a readily
available lone pair of electrons which can participate in bonding. Primary and
sec-ondary amines can act as weak electrophiles or acids with a strong base, by
losing an N–H proton to form an amide anion (R2N-).
Spectroscopic analysis
Evidence for primary and secondary amines include N–H stretching
and bending absorptions in the IR spectrum as well as a D2O
exchangeable proton in the 1H nmr spectrum.
Structure
Amines consist of an sp3 hybridized nitrogen linked to three substituents by
three bonds. The substituents can be hydrogen, alkyl, or aryl groups, but at
least one of the substituents has to be an alkyl or aryl group. If only one
such group is present, the amine is defined as primary. If two groups are
present, the amine is secondary. If three groups are present, the amine is
tertiary. If the substituents are all alkyl groups, the amine is defined as
being an alkylamine. If there is at least one aryl group directly attached to
the nitrogen, then the amine is defined as an arylamine.
The nitrogen atom has four sp3 hybridized orbitals pointing to the corners of a
tetrahedron in the same way as ansp3
hybridized carbon atom. However, one of the sp3
orbitals is occupied by the nitrogen’s lone pair of electrons. This means that
the atoms in an amine functional group are pyramidal in shape. The C–N–C bond
angles are approximately 109° which
is consistent with a tetrahedral nitrogen. However, the bond angle is slightly
less than 109° since the lone pair of electrons demands a
slightly greater amount of space than a σ bond.
Pyramidal inversion
Since amines are tetrahedral, they are chiral if they have three different substituents. However, it is not possible to separate the enantiomers of a chiral amine since amines can easily undergo pyramidal inversion – a process which interconverts the enantiomers (Fig. 1). The inversion involves a change of hybridization where the nitrogen becomes sp2 hybridized rather than sp3hybridized. As a result, the molecule becomes planar and the lone pair of elec-trons occupy a p orbital. Once the hybridization reverts back to sp3, the molecule can either revert back to its original shape or invert.
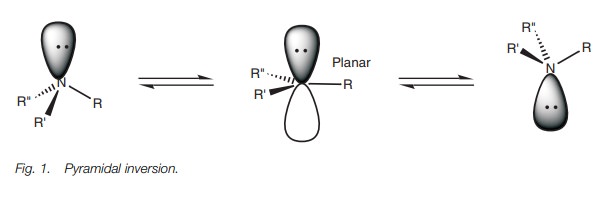
Although the enantiomers of chiral amines
cannot be separated, such amines can be alkylated to form quaternary ammonium
salts where the enantiomers can be
separated. Once the lone pair of electrons is locked up in aσ bond, pyramidal inversion becomes impossible and the enantiomers
can no longer interconvert.
Physical properties
Amines are polar compounds and intermolecular
hydrogen bonding is possible for primary and secondary amines. Therefore,
primary and secondary amines have higher boiling points than alkanes of similar
molecular weight. Tertiary amines also have higher boiling points than
comparable alkanes, but have slightly lower boiling points than comparable
primary or secondary amines since they cannot take part in intermolecular
hydrogen bonding.
However, all amines can participate in hydrogen
bonding with protic solvents, which means that amines have similar water
solubilities to comparable alcohols.
Low molecular weight amines are freely miscible
with water. Low molecular weight amines have an offensive fish-like odor
Basicity
Amines are weak bases but they are more basic
than alcohols, ethers, or water. As a result, amines act as bases when they are
dissolved in water and an equilibrium is set up between the ionized form (the ammonium ion) and the unionized form
(the free base; Fig. 2).
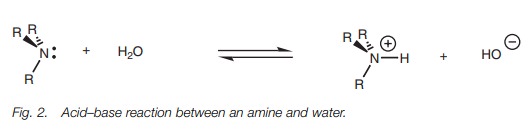
The basic strength of an amine can be measured
by its pKb value. The
lower the value of pKb,
the stronger the base. The pKb
for ammonia is 4.74, which compares with pKb
values for methylamine, ethylamine, and propy-lamine of 3.36, 3.25 and 3.33,
respectively. This demonstrates that larger alkyl groups increase base
strength. This is an inductive effect whereby the ion is stabi-lized by
dispersing some of the positive charge over the alkyl group (Fig. 3). This shifts the equilibrium of
the acid base reaction towards the ion, which means that the amine is more
basic. The larger the alkyl group, the more significant this effect.
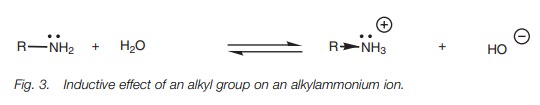
Further alkyl substituents should have an even
greater inductive effect and one might expect secondary and tertiary amines to
be stronger bases than primary amines. This is not necessarily the case and
there is no direct relationship between basicity and the number of alkyl groups
attached to nitrogen. The inductive effect of more alkyl groups is counterbalanced
by a solvation effect.
Once the ammonium ion is formed, it is solvated
by water molecules – a stabilizing factor which involves hydrogen bonding
between the oxygen atom of water and any N–H group present in the
ammonium ion (Fig. 4). The more
hydro-gen bonds which are possible, the greater the stabilization. As a result,
solvation and solvent stabilization is stronger for alkylaminium ions formed
from primary amines than for those formed from tertiary amines. The solvent
effect tends to be more important than the inductive effect as far as tertiary
amines are concerned and so tertiary amines are generally weaker bases than
primary or secondary amines.
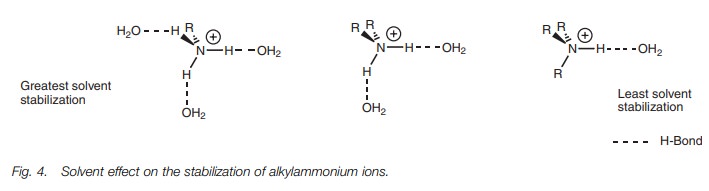
Aromatic amines (arylamines) are weaker bases
than alkylamines since the orbital containing nitrogen’s lone pair of electrons
overlaps with the π system of the aromatic ring. In terms of
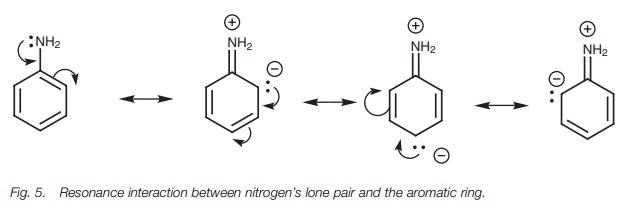
resonance, the lone pair of electrons can be used to form a double bond to the
aromatic ring, resulting in the possibility of three zwit-terionic resonance structures (Fig. 5). (A zwitterion is a molecule containing a pos-itive and a
negative charge.) Since nitrogen’s lone pair of electrons is involved in this
interaction, it is less available to form a bond to a proton and so the amine
is less basic.
The nature of aromatic substituent also affects
the basicity of aromatic amines. Substituents which deactivate aromatic rings
(e.g. NO2, Cl, or CN) lower electron density in the ring, which
means that the ring will have an electron-withdrawing effect on the neighboring
ammonium ion. This means that the charge will be destabilized and the amine
will be a weaker base. Substituents which activate the aromatic ring enhance
electron density in the ring which means that the ring will have an
electron-donating effect on the neighboring charge. This has a stabilizing
effect and so the amine will be a stronger base. The relative position of
aromatic substituents can be important if resonance is possible between the
aromatic ring and the substituent. In such cases, the substituent will have a
greater effect if it is at the ortho
or para position. For example, para-nitroaniline is a weaker base than meta-nitroaniline. This is because one
of the possible resonance structures for the
para isomer is highly disfavored since it places a positive charge
immediately nextto the ammonium ion (Fig.
6). Therefore, the number of feasible resonance struc-tures for the para isomer is limited to three,
compared to four for the meta isomer.
This means that the para isomer
experiences less stabilization and so the amine will be less basic.
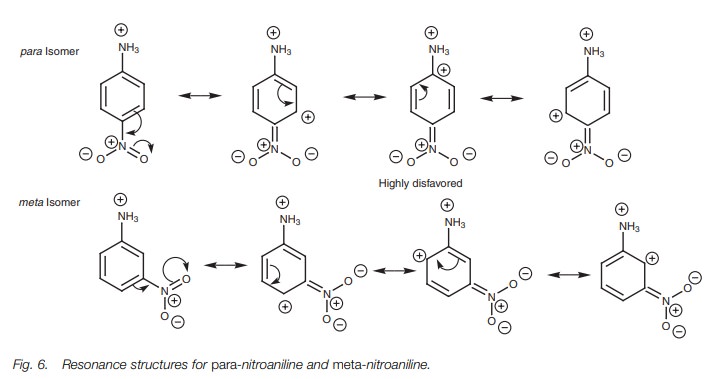
If an activating substituent is present, capable of interacting with the ring by resonance, the opposite holds true and the para isomer will be a stronger base than the meta isomer. This is because the crucial resonance structure mentioned above would have a negative charge immediately next to the ammonium ion and this would have a stabilizing effect..
Reactivity
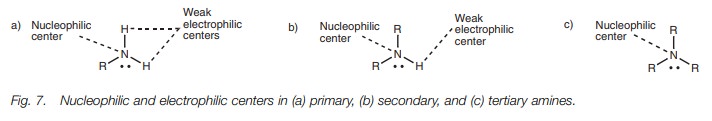
Amines react as nucleophiles or bases, since
the nitrogen atom has a readily available lone pair of electrons which can
participate in bonding (Fig. 7). As a
result, amines react with acids to form water soluble salts. This allows the
easy separation of amines from other compounds. A crude reaction mixture can be
extracted with dilute hydrochloric acid such that any amines present are
protonated and dissolve into the aqueous phase as water-soluble salts. The free
amine can be recovered by adding sodium hydroxide to the aqueous solution such
that the free amine precipitates out as a solid or as an oil.
Amines will also react as nucleophiles with a
wide range of electrophiles includ-ing alkyl halides, aldehydes, ketones, and
acid chlorides.
The N–H protons of primary and secondary
amines are weakly electrophilic or acidic and will react with a strong base to
form amide anions. For example, diiso-propylamine (pKa ~40) reacts with
butyllithium to give lithium diisopropylamide (LDA) and butane.
Spectroscopic analysis
Primary and secondary amines are likely to show
characteristic absorptions due to
N–H stretching and
N–H bending. The
former occurs in
the region 3500–3300 cm−1,
and in the case of primary amines two absorptions are visible. The absorptions
tend to be sharper but weaker than O–H absorptions which can occur in the same
region. N–H bending occurs in the region 1650–1560 cm−1 for primary
amines and 1580–1490 cm−1 for
secondary amines although the latter tend to be weak and unreliable. These
absorptions occur in the same region as alkene and aromatic C=C stretching absorptions,
and care has to be taken in assigning them.
Naturally, these absorptions are not present
for tertiary amines. For aromaticamines,
an absorption due
to Ar–N stretching
may be visible
in the region1360–1250 cm−1.
The 1H nmr spectrum of a primary or
secondary amine will show a broad sig- nal for the N–H proton in the region
0.5–4.5 ppm which will disappear from the spectrum if the sample is shaken with
deuterated water. For aromatic amines this signal is typically in the range 3–6
ppm. The chemical shifts of neighboring groups can also indicate the presence
of an amine group indirectly. For example, an N-methyl group gives a singlet
near 2.3 ppm in the 1H spectrum and appears in the region 30–45 ppm in the 13C
spectrum.
If the molecular ion in the mass spectrum has
an odd number, this indicates that an odd number of nitrogen atoms are present
in the molecule. This supports the presence of an amine but does not prove it,
since there are other functional groups containing nitrogen. Amines undergo
α-cleavage when they fragment (i.e. cleavage next to the carbon bearing the
amine group.
Related Topics