Chapter: Organic Chemistry: Amines and nitriles
Reactions of amines
REACTIONS OF AMINES
Key Notes
Alkylation
Ammonia,
primary amines, and secondary amines can be alkylated with alkyl halides to
give primary, secondary, and tertiary amines, respectively. However,
over-alkylation usually occurs and mixtures are obtained. The method is best
used for converting tertiary amines to quaternary ammo-nium salts. A better
method of alkylating a primary or secondary amine is to treat the amine with an
aldehyde or ketone in the presence of a reducing agent. Reaction of the amine
with the carbonyl compound produces an intermediate imine which is reduced to
the amine. No over-alkylation takes place.
Acylation
Primary
and secondary amines can be acylated with an acid chloride or acid anhydride to
give secondary and tertiary amides, respectively.
Sulfonylation
Primary
and secondary amines can be sulfonylated with a sulfonyl chloride to give a
sulfonamide.
Elimination
Primary
amines can be converted to alkenes if the amine is first methylated to a
quaternary ammonium salt, then treated with silver oxide. Elimination of
triethylamine takes place to give the least substituted alkene. The reac-tion
is known as the Hofmann elimination. The reaction can also be carried out on
secondary and tertiary amines although a mixture of alkenes may be formed
depending on the substituents present. Aromatic amines will also react if they
contain a suitable N-alkyl
substituent.
Electrophilic aromatic substitution
Aromatic
amines undergo electrophilic aromatic substitutions. The amino group is
strongly activating and directs substitution to the ortho and para positions.
Nitration, sulfonation, and bromination are all possible, but bromination may
occur more than once. Friedel–Crafts alkylation and acylation are not possible
since the amino group complexes the Lewis acid involved in the reaction. The
problems of excess bromination and Lewis acid complexation can be overcome by
converting the amine to an amide before carrying out the substitution reaction.
The amide can be hydrolyzed back to the amine once the substitution reaction
has been carried out.
Diazonium salts
Aromatic
primary amines can be converted to diazonium salts on treatment with nitrous
acid. These salts are extremely important in aromatic chemistry since they can
be converted to a variety of other substituents. Diazonium salts also react
with phenols or aromatic amines in a process called diazo-nium coupling to
produce a highly conjugated system which is usually colored. Such products are
often used as dyes.
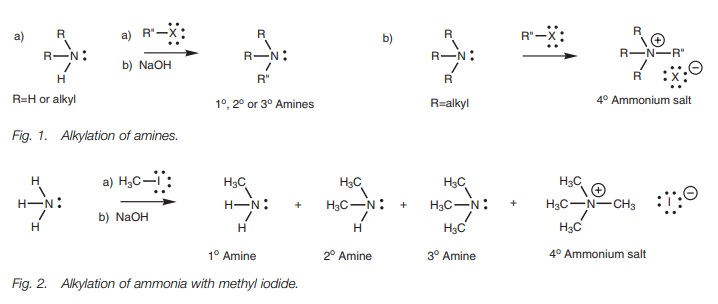
Alkylation
Ammonia, primary amines, and secondary amines
(both aromatic and aliphatic) can undergo the SN2 reaction with
alkyl halides to produce a range of primary, secondary, and tertiary amines.
Primary, secondary, and tertiary amines are produced as ammonium salts which
are converted to the free amine by treat-ment with sodium hydroxide (Fig. 1a).
In theory, it should be possible to synthesize
primary amines from ammonia, secondary amines from primary amines, and tertiary
amines from secondary amines. In practice, over-alkylation is common. For
example, reaction of ammonia with methyl iodide leads to a mixture of primary,
secondary, and tertiary amines along with a small quantity of the quaternary
ammonium salt (Fig. 2).
Alkylation of tertiary amines by this method is
a good way of obtaining quaternary ammonium salts (Fig. 1b) since no other products are possible. However, alkylation
of lower order amines is not so satisfactory.
A better method of alkylating a primary or secondary amine is to treat the amine with a ketone or an aldehyde in the presence of a reducing agent – sodium cyanoborohydride. This reaction is known as a reductive amination. Over-alkylation cannot occur by this method.
Acylation
Primary and secondary amines (both aromatic and
aliphatic) can be acylated with an
acid chloride or
acid anhydride to form
secondary and tertiary
amides, respectively. This reaction can be viewed as the acylation of an
amine or as the nucleophilic substitution of a carboxylic acid derivative.
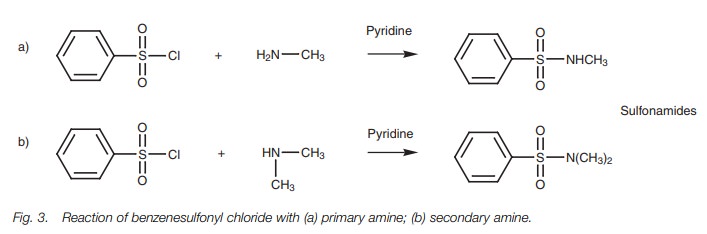
Sulfonylation
In a similar reaction to acylation, primary and
secondary amines (both aromatic and aliphatic) can be treated with a sulfonyl
chloride to give a sulfonamide (Fig.3).
Tertiary amines do not give a stable product and are recovered unchanged.
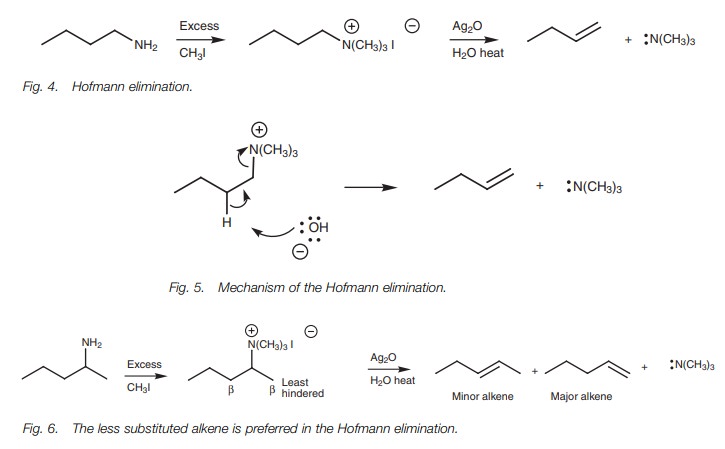
Elimination
Primary amines could be converted to alkenes if
it was possible to eliminate ammonia from the molecule. However, the direct
elimination of ammonia is not possible. A less direct method of achieving the
same result is to exhaustively methylate the amine by the SN2
reaction to give a quaternary ammonium salt. Once this is formed, it is
possible to eliminate triethylamine in the presence of silver oxide and to form
the desired alkene. The reaction is called the Hofmann elimination (Fig. 4).
The silver oxide provides a hydroxyl ion which acts as the base for an E2
elimination. However, unlike most E2 eliminations, the less substituted alkene
is preferred if a choice is available (Fig.
6). The reason for this preference is not fully understood, but may have
something to do with the large bulk of the triethylamine leaving group
hindering the approach of the hydroxide ion such that it approaches the least
hindered β-carbon.\
Secondary and tertiary amines can also be
exhaustively methylated then treated with silver oxide. However, mixtures of
different alkenes may be obtained if the N-substituents
are different alkyl groups (Fig. 7).
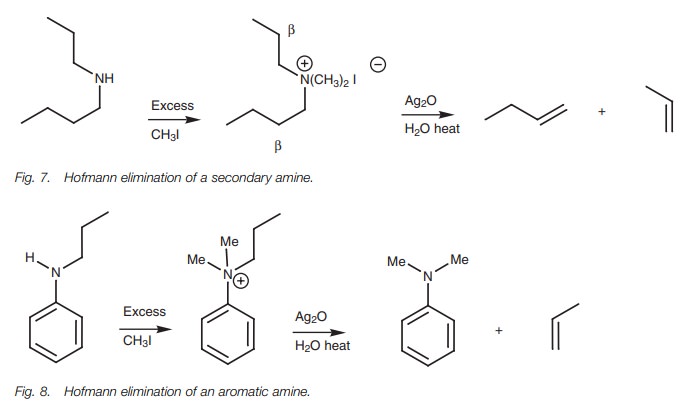
The Hofmann elimination is not possible with
primary arylamines, but sec-ondary and tertiary arylamines will react if one of
the substituents is a suitable alkyl group. Elimination of the aromatic amine
can then occur such that the alkyl substituent is converted to the alkene (Fig. 8).
Electrophilic aromatic substitution
Aromatic
amines such as
aniline undergo electrophilic
substitution reactions where the
amino group acts as a strongly activating group, directing substitution to the ortho and para positions. Like phenols, the amino group is such a strong
activating group that more than one substitution may take place. For example,
reaction of aniline with bromine results in a tribrominated structure as the
only product. This problem can be overcome by converting the amine to a less
acti-vating group. Typically, this involves acylating the group to produce an
amide. This group is a weaker activating group and so mono-substitution takes
place. Furthermore, since the amide group is bulkier than the original amino
group, there is more of a preference for para
substitution over ortho substitution.
Once the reaction has been carried out, the amide can be hydrolyzed back to the
amino group .
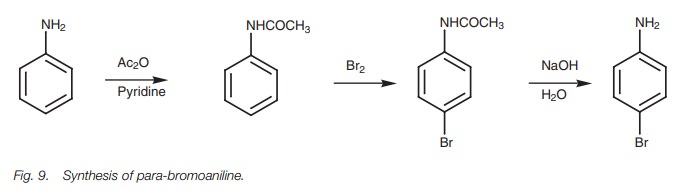
Anilines can be sulfonated and nitrated, but
the Friedel–Crafts alkylation and acylation are not possible since the amino
group forms an acid base complex with the Lewis acid required for this
reaction. One way round this is to convert the aniline to the amide as above
before carrying out the reaction.
Diazonium salts
Primary arylamines or anilines can be converted
to diazonium salts, which in turn can be converted to a large variety of
substituents (Fig. 10).

Reaction of an aniline with nitrous acid
results in the formation of the stable diazonium salt in a process called diazotization (Fig. 11). In the strong acid condi-tions used, the nitrous acid
dissociates to form an +NO ion which can then act as an electrophile.
The aromatic amine uses its lone pair of electrons to form a bond to this +NO
ion. Loss of a proton from the intermediate formed, followed by a pro-ton shift
leads to the formation of a diazohydroxide. The hydroxide group is now
protonated turning it into a good leaving group, and a lone pair from the aryl
nitrogen forms a second π bond between the two nitrogen atoms and expels
water.
Once the diazonium salt has been formed, it can be treated with various nucleo-philes such as Br- , Cl- , I- , -CN and -OH (Fig. 12). The nucleophile displaces nitrogen from the aromatic ring and the nitrogen which is formed is lost from the reaction mixture as a gas, thus helping to drive the reaction to completion. Those reactions involving Cu(I) are also known as the Sandmeyer reaction.
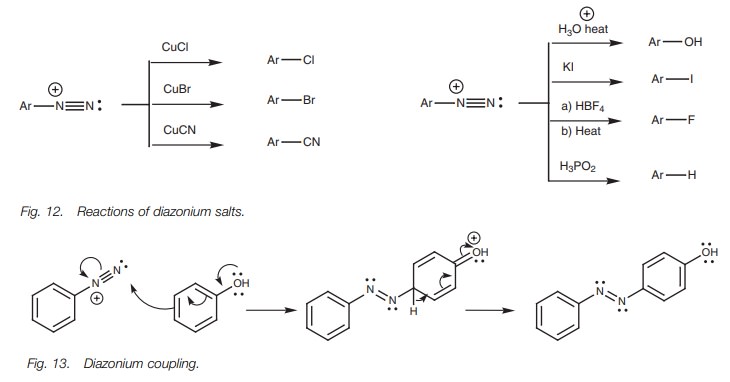
Diazonium salts are also used in a reaction
called diazonium coupling where the
diazonium salt is coupled to the para
position of a phenol or an arylamine (Fig.13).
The azo products obtained have an extended conjugated system whichincludes both
aromatic rings and the N=N link. As a result, these compounds are often colored
and are used as dyes.
The above coupling is more efficient if the
reaction is carried out under slightly alkaline conditions (NaOH) such that the
phenol is ionized to a phenoxide ion (ArO- ). Phenoxide ions are
more reactive to electrophilic addition than phenols themselves. Strong
alkaline conditions cannot be used since the hydroxide ion adds to the
diazonium salt and prevents coupling. If the para position of the phe-nol is already occupied, diazo coupling
can take place at the ortho position
instead.
Aliphatic amines, as well as secondary and
tertiary aromatic amines, react with nitrous acid, but these reactions are less
useful in organic synthesis.
Related Topics