Chapter: Plant Biochemistry: Lipids are membrane constituents and function as carbon stores
Polar lipids are important membrane constituents
Polar lipids are important membrane constituents
The polar glycerolipids are amphiphilic molecules, consisting of a hydrophilic head and a hydrophobic tail. This property enables them to form lipid bilay-ers, in which the hydrocarbon tails are held together by hydrophobic inter-actions and the hydrophilic heads protrude into the aqueous phase, thus forming the basic structure of a membrane (bilayer) (Fig. 15.2). Since the middle C atom of the glycerol in a polar glycerolipid is asymmetric, a distinc-tion can be made between the two esterified groups of glycerol at the C-1-and C-3-positions.
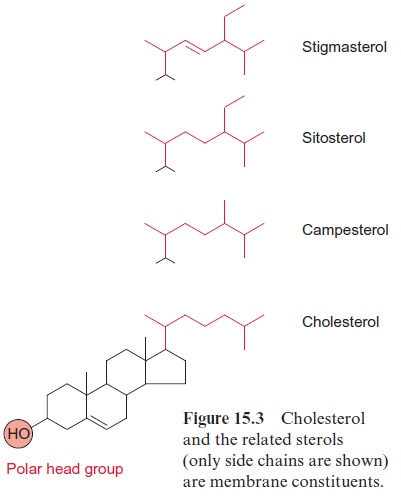
Other membrane lipids in plants are sphingolipids (Fig. 15.5C) which are important constituents of plasma membranes. The sterols shown in Figure 15.3 are also amphiphilic, the hydroxyl groups form the hydrophilic head and the sterane skeleton with the side chain serves as the hydrophobic tail. In addition to the sterols shown here, plants contain a large variety of other sterols as membrane constituents, many of them are present in the outer membrane of mitochondria, in the membranes of the endoplasmatic reticu-lum, and in the plasma membrane. Sterols determine to a large extent the properties of these membranes (see below).
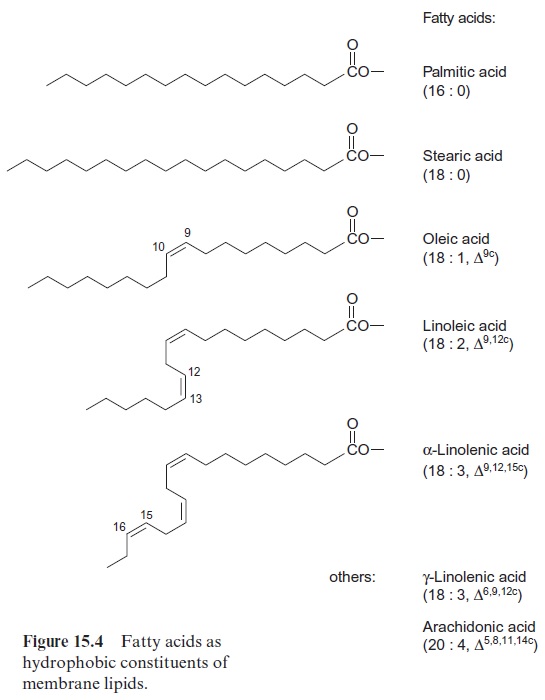
The polar glycerolipids are comprised mainly of fatty acids with 16 or 18 carbon atoms (Fig. 15.4). The majority of these fatty acids are unsatu-rated and contain one to three carbon-carbon double bonds. These dou-ble bonds are almost exclusively in the cis-configuration and rarely in the trans-configuration. The double bonds are usually not conjugated. Figure 15.4 shows the number code for the structure of fatty acids: number of C atoms, number of double bonds, ∆-position of the first C atom of the dou-ble bond, c= cis-configuration (t ==trans).
The storage lipids of plants often contain unusual fatty acids (e.g., with conjugated double bonds, carbon-carbon triple bonds, or hydroxyl-, keto-or epoxy groups). By now more than 500 of these unusual fatty acids are known. There are also glycerolipids, in which the carbon chain is connected with the glycerol via an ether linkage.
The fluidity of the membrane is governed by the proportion of unsaturated fatty acids and the content of sterols
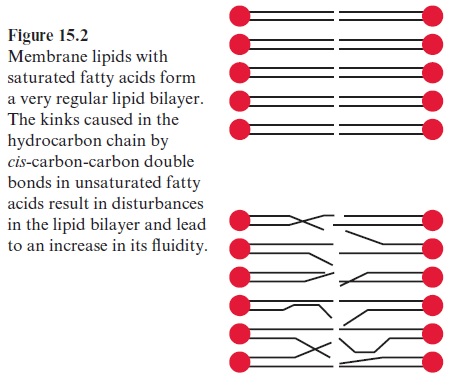
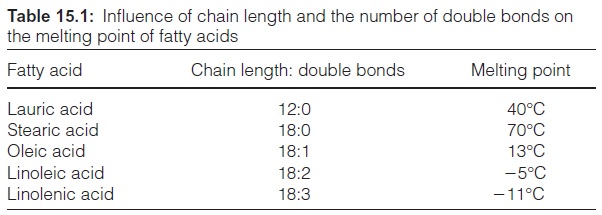
The hydrocarbon chains in saturated fatty acids are packed in a regular bilayer (Fig. 15.2), whereas in unsaturated fatty acids the packing is dis-turbed due to kinks in the hydrocarbon chain, caused by the cis-carbon-carbon double bonds, resulting in a more fluid layer and having an effect on the melting points of various fatty acids (Table 15.1). The melting point increases with increasing chain length as the packing becomes tighter, whereas the melting point decreases with an increasing number of double bonds. This feature also applies to the corresponding fats. Cocoa fat, for instance, consisting only of saturated fatty acids, is solid at room tempera-ture, whereas plant oils, with a very high natural content of unsaturated fatty acids, are liquid.

Likewise, the fluidity of membranes is governed by the proportion of unsaturated fatty acids in the membrane lipids. This is why in some plants, during growth at a low temperature, more highly unsaturated fatty acids are incorporated into the membrane to compensate for the decrease in membrane fluidity. It has been demonstrated that it is possible to enhance the cold tolerance of tobacco by increasing the proportion of unsaturated fatty acids in the membrane lipids by genetic engineering. Sterols (Fig. 15.3) decrease the fluidity of membranes and probably also play a role in the adaptation of membranes to temperature. On the other hand, a decrease of unsaturated fatty acids in the lipids of thylakoid membranes, as achieved by genetic engineering, made tobacco plants more tolerant to heat.
Membrane lipids contain a variety of hydrophilic head groups
The head groups of the polar glycerolipids, which provide the lipid mol-ecule with a polar group, are formed in plants by a variety of compounds (Fig. 15.5). In thephospholipids, the head group consists of a phosphate residue that is esterified with a second alcoholic compound such as eth-anolamine, choline, serine, glycerol, or inositol. Phosphatidic acid is only a minor membrane constituent, but it plays a role as a signaling compound .
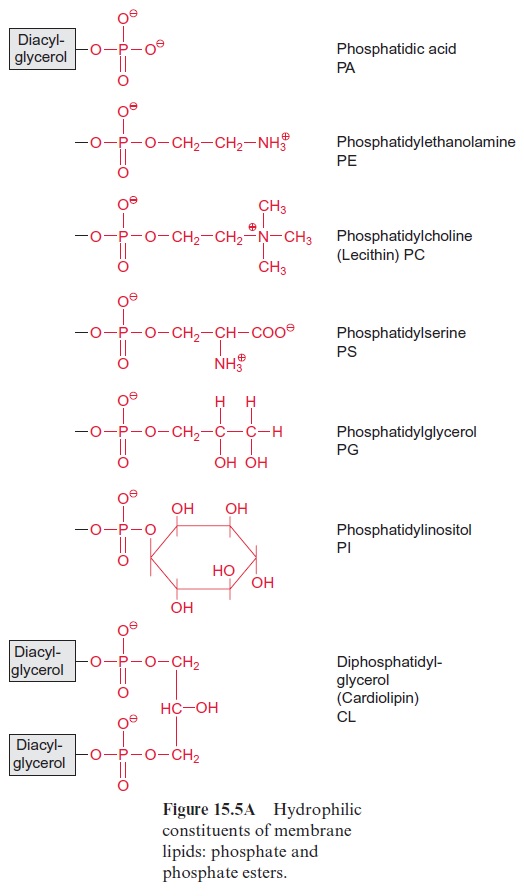
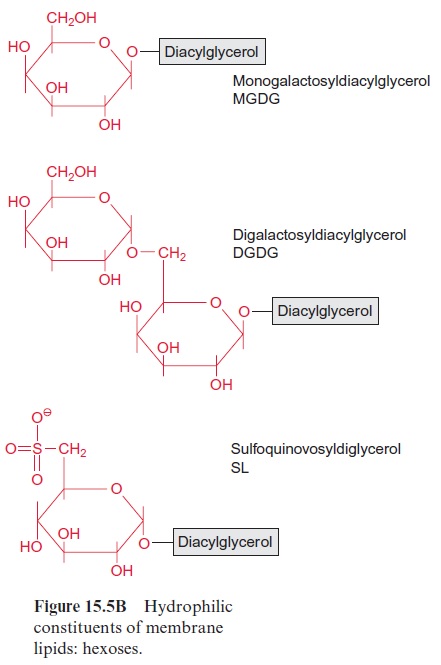
The phospholipids are found as membrane constituents in bacteria as well as in animals and plants. As a specialty of plants and cyanobacteria the galactolipidsmonogalactosyldiacylglycerol (MGDG) and digalactosyl-diacylglycerol (DGDG), and the sulfolipid sulfoquinovosyldiacylglycerol (SL) are additionally present in the membranes. In SL a glucose moiety to which a sulfonic acid residue is linked at the C-6-position forms the polar head group (Fig. 15.5B).
There are great differences in the lipid composition of the various mem-branes in a plant (Table 15.2). The main constituents of the chloroplast thylakoid and envelope membranes are galactolipids. The membranes of the mitochondria and the plasma membrane do not contain galactolipids but have phospholipids as the main membrane constituents. Cardiolipin is a specific component of the inner mitochondrial membrane in animals and plants (Fig. 15.5A).
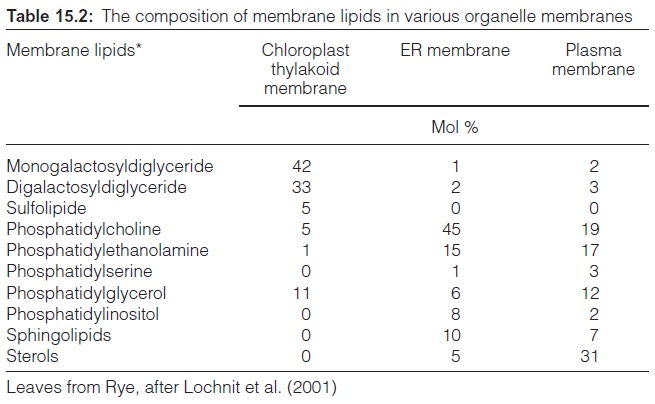
In a green plant cell, about 70% to 80% of the total membrane lipids are constituents of the thylakoid membranes. Plants represent the largest part of the biosphere, and this is why the galactolipids MGDG and DGDG are the most abundant membrane lipids on earth. In many habitats plant growth is limited by the phosphate content in the soil, therefore it was prob-ably advantageous during evolution for plants to synthesize—independent of the phosphate supply of the soil—galactolipids rather than phospholip-ids as dominant membrane lipids.
Sphingolipids are important constituents of the plasma membrane
Sphingolipids (Fig. 15.5C) are present in the plasma and ER membranes. The sphingolipids consist of a so-called sphingo base. This is a hydrocarbon chain containing double bonds, an amino group in position 2, and two to three hydroxyl groups in positions 1, 3, and 4. The sphingo base is con-nected by an amide link to a fatty acid (C16–24, with up to two double bonds). As an example of one of the many sphingolipids, ceramide, with sphinganine as base, is shown in Figure 15.5C. Inglucosylsphingolipids, the terminal hydroxyl group is linked to a glucose residue, whereas phosphoryl sphingolipids (not shown in Fig. 15C) are esterified with phosphate or phosphocholine.
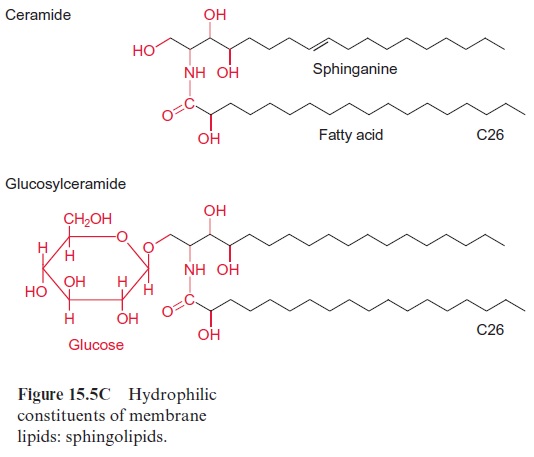
Sphingolipids are known to have an important signaling function in ani-mals and yeast. Research on the function of sphingolipids in plants is still in its infancy. Recent results indicate that sphinganine 1-phosphate acts in guard cells as a Ca++ -mobilizing messenger, which is released upon the action of abscisic acid (ABA). Plant sphin-golipid metabolites may be also involved as signals in programmed cell death.
Related Topics