Chapter: Plant Biochemistry: Lipids are membrane constituents and function as carbon stores
Glycerol 3-phosphate is a precursor for the synthesis of glycerolipids
Glycerol 3-phosphate is a precursor for the synthesis of glycerolipids
Glycerol 3-phosphate is synthesized by reduction of dihydroxyacetone phosphate with NADH as reductant (Fig. 15.18). Dihydroxyacetone phos-phate reductases are present in the plastid stroma as well as in the cytosol. In plastid lipid biosynthesis, the acyl residues are transferred directly from acyl ACP to glycerol 3-phosphate. For the first acylation step, mostly an 18:1-, less frequently a 16:0-, and more rarely an 18:0-acyl residue is esteri-fied to carbon position 1 of glycerol 3-phosphate. The C-2-position, how-ever, is always esterified with a 16:0-acyl residue. Since this specificity is also observed in cyanobacteria, the glycerolipid biosynthesis pathway of the plastids is called the prokaryotic pathway.
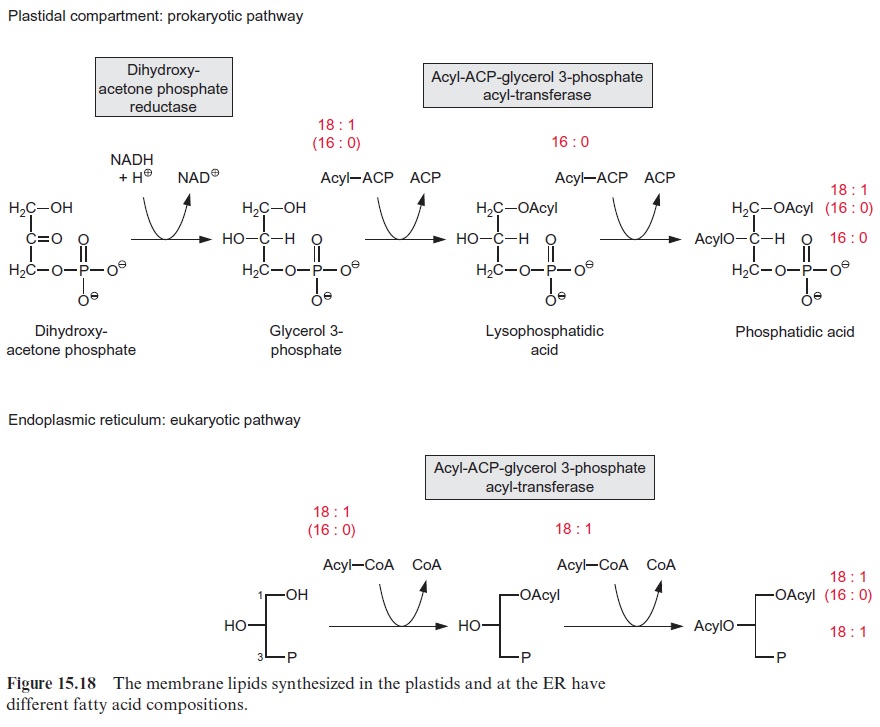
For glycerolipid synthesis in the ER membrane, the acyl residues are trans-ferred from acyl CoA. Here again, the hydroxyl group in the C-1-position of glycerol 3-phosphate is esterified with an 18:1-, 16:0-, or 18:0-acyl residue, and position C-2 is always linked with a desaturated 18:n-acyl residue. The glycer-olipid pathway of the ER membrane is called the eukaryotic pathway.
For glycerolipid synthesis in the ER membrane, the acyl residues are trans-ferred from acyl CoA. Here again, the hydroxyl group in the C-1-position of glycerol 3-phosphate is esterified with an 18:1-, 16:0-, or 18:0-acyl residue, and position C-2 is always linked with a desaturated 18:n-acyl residue. The glycer-olipid pathway of the ER membrane is called the eukaryotic pathway.
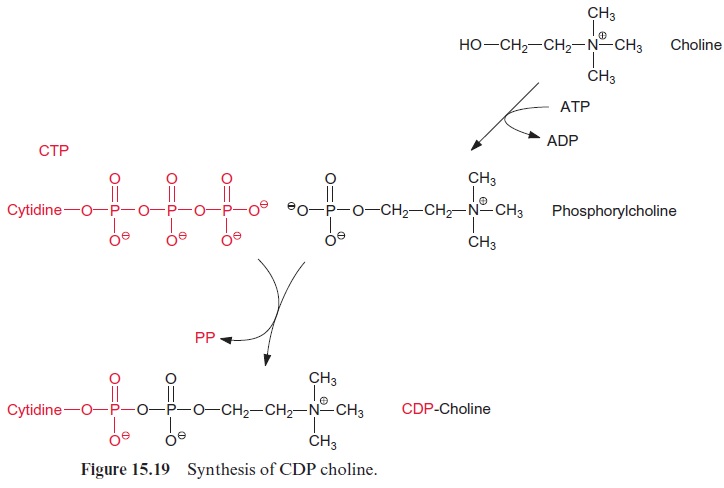
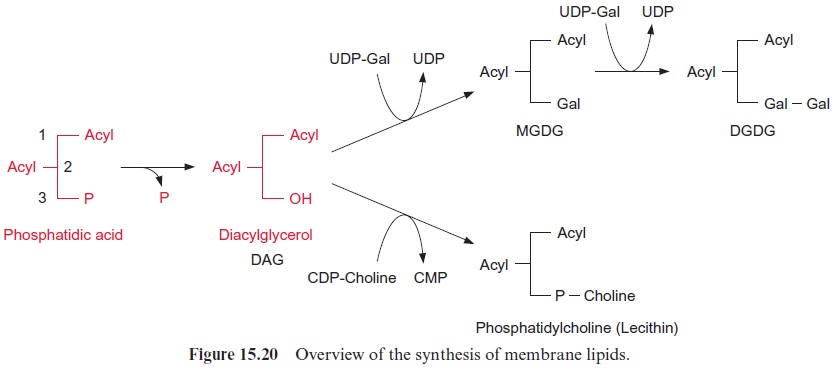
The linkage of the polar head group to diacylglycerol proceeds mostly via activation of the head group, but in some cases also by an activation of diacylglycerol. Choline and ethanolamine are activated by phosphoryla-tion via specific kinases and are then converted via cytidyl transferases by reaction with CTP to CDP choline and CDP ethanolamine (Fig. 15.19). A galactose head group is activated as UDP galactose (Fig. 15.20). The lat-ter is synthesized from glucose 1-phosphate and UTP via the UDP glucose pyrophosphorylase and UDP glucose epimerase (Fig. 9.21). For the synthesis of digalactosyldiacylglycerol (DGDG) from monogalacto syldiacylglycerol (MGDG), a galactose residue is transferred from UDP galactose. Also, sulfoquinovose is activated as a UDP derivative, but details of the synthesis of this moiety will not be discussed here. The acceptor for the activated head group is diacylglycerol, which is formed from phospha-tidic acid by the hydrolytic release of the phosphate residue.
The ER membrane is the site of fatty acid elongation and desaturation
As shown in Figure 15.17, the plastids produce 16:0-, 18:1-, and to a lesser extent 18:0-acyl residues. However, some storage lipids contain fatty acids with longer chains. This also applies to waxes, which are esters of long chain fatty acid (C20–C24) and very long chain acyl alcohols (C24–C32). The elongation of fatty acids longer than C18 is catalyzed by elongases, which are located in the membranes of the ER (Fig. 15.21). Elongation proceeds in the same way as fatty acid synthesis, with the differences that other enzymes are involved and the acyl and malonyl residues are activated as acyl CoA thioesters.
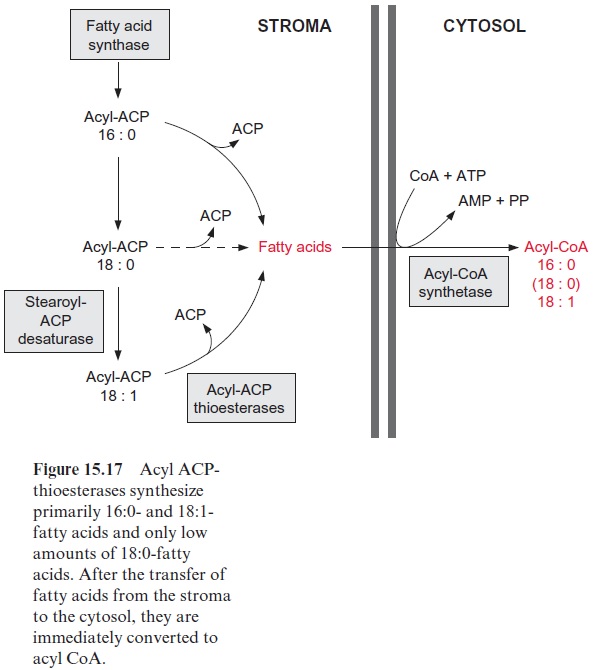
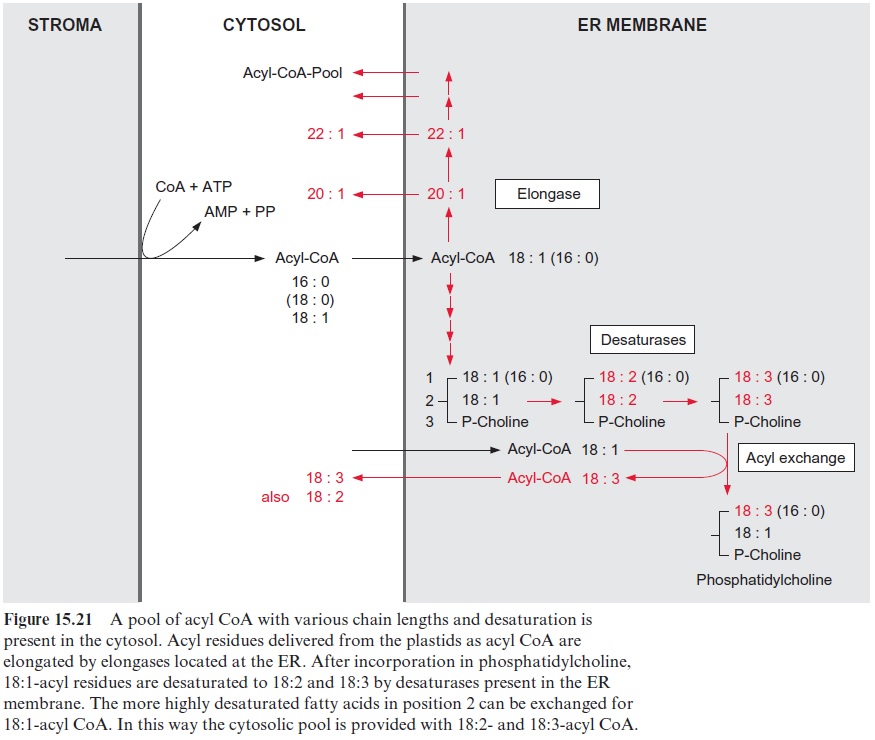
The ER membrane is also the site for further desaturations of the acyl residue. For desaturation, the acyl groups are first incorporated into phos-pholipids, such as phosphatidylcholine (Fig. 15.21). Desaturases bound to the membrane of the ER convert oleate (18:1) to linoleate (18:2) and then to linolenate (18:3). The 18:2- or 18:3-acyl residues in the C-2-position of glycerol can be exchanged for an 18:1-acyl residue, and the latter can then be further desaturated.
The interplay of the desaturases in the plastids and the ER provides the cell with an acyl CoA pool to cover the various needs of the cytosol. The 16:0-, 18:0-, and 18:1-acyl residues for this pool are delivered by the plas-tids, and the longer chains and more highly unsaturated acyl residues are provided through modifications by the ER membrane.
Some of the plastid membrane lipids are synthesized via the eukaryotic pathway
The synthesis of glycerolipids destined for the plastid membranes occurs in the envelope membranes of the plastids (Fig. 15.22). Besides the prokaryo-tic pathway of glycerolipid synthesis, in which the acyl residues are directly transferred from acyl ACP, glycerolipids are also synthesized via the eukaryotic pathway. In the latter case, the desaturases of the ER membrane can provide double unsaturated fatty acids for plastid membrane lipids. A precursor is, for instance, phosphatidylcholine with double unsaturated fatty acids, which has been synthesized in the ER membrane (Fig. 15.21). This phosphatidylcholine is transferred to the envelope membrane of the plastids and hydrolyzed to diacylglycerol and then substituted with a head group consisting of one or two galactose residues. The acyl residues can be further desaturated to 18:3 by a desaturase present in the envelope membrane.
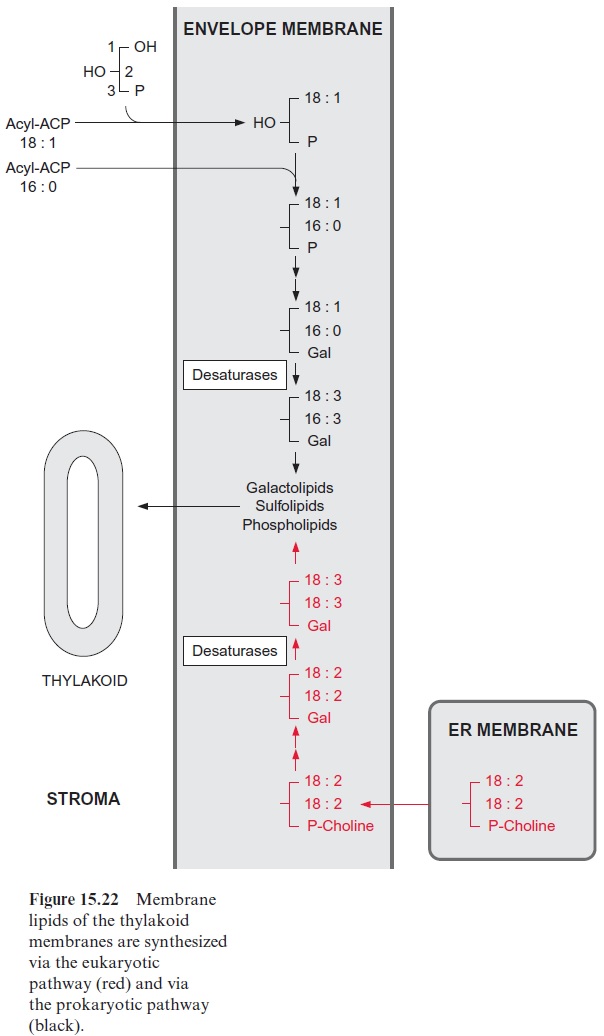
Some desaturases in the plastid envelope are able to desaturate lipid-bound 18:1- and 16:0-acyl residues. A comparison of the acyl residues in the C-2-position (in the prokaryotic pathway 16:0 and in the eukaryo-tic pathway 18:n) demonstrated, however, that a large proportion of the highly unsaturated galactolipids in the plastids are synthesized via a detour through the eukaryotic pathway. The membrane lipids of the envelope membranes are probably transferred by a special transfer protein to the thy-lakoid membrane.
Related Topics