Chapter: Medical Surgical Nursing: Assessment of Renal and Urinary Tract Function
Physiology of the Upper and Lower Urinary Tracts
PHYSIOLOGY
OF THE UPPER AND LOWER URINARY TRACTS
The
urinary system performs various roles that are essential for nor-mal bodily
homeostasis (Chart 43-1). These functions include urine formation; excretion of
waste products; regulation of electrolyte, acid, and water excretion; and
autoregulation of blood pressure.
Urine Formation
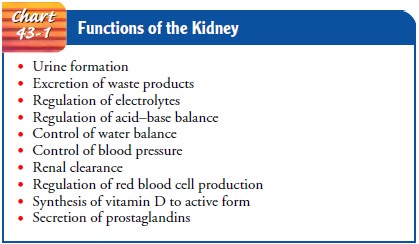
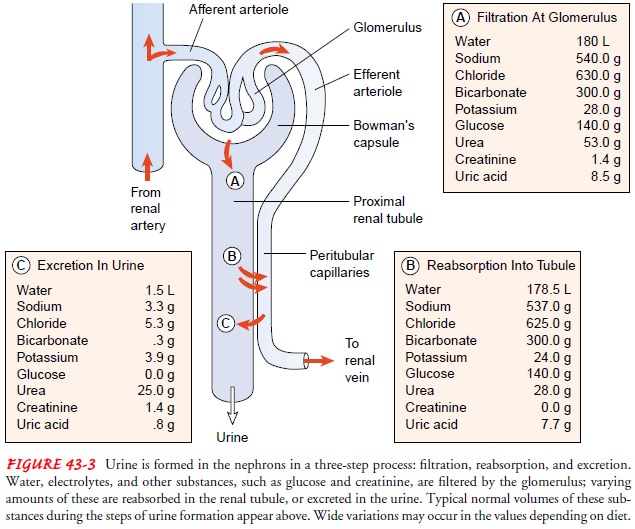
Urine is formed in the nephrons through a complex three-step process: glomerular filtration, tubular reabsorption, and tubu-lar secretion. Figure 43-3 illustrates the three processes of urineformation and typical values of water and electrolytes associated with each process. The various substances normally filtered by the glomerulus, reabsorbed by the tubules, and excreted in the urine include sodium, chloride, bicarbonate, potassium, glucose, urea, creatinine, and uric acid. Within the tubule, some of these sub-stances are selectively reabsorbed into the blood. Others are se-creted from the blood into the filtrate as it travels down the tubule. Some substances, such as glucose, are completely re-absorbed in the tubule and normally do not appear in the urine. Amino acids and glucose are usually filtered at the level of the glomerulus and reabsorbed so that neither is excreted in the urine.
Glucose, however,
appears in the urine (glycosuria) if the amount of glucose in the blood and
glomerular filtrate exceeds the amount that the tubules are able to reabsorb.
Normally, glucose is completely reabsorbed when the blood glucose level is less
than 200 mg/dL (11 mmol/L). In diabetes, when the blood glucose level exceeds
the kidneys’ reabsorption capacity, glucose appears in the urine. Glycosuria is
also common in pregnancy.
Protein molecules are also generally not found in the urine; however, low-molecular-weight proteins (globulins and albumin) may periodically be excreted in small amounts. Transient pro-teinuria in amounts less than 150 mg/dL is considered normaland does not require further evaluation. Persistent proteinuria usually signifies damage to the glomeruli.
The
steps of urine formation are:
Glomerular
filtration: The normal blood flow through the kidneys is about 1,200 mL/min. As
blood flows into the glomerulus from an afferent arteriole, filtration occurs.
The filtered fluid, also known as filtrate or ultrafiltrate, then enters the renal
tubules. Under normal conditions, about 20% of the blood passing through the
glomeruli is fil-tered into the nephron, amounting to about 180 L/day of
fil-trate. The filtrate normally consists of water, electrolytes, and other
small molecules, because water and small molecules are allowed to pass, whereas
larger molecules stay in the blood-stream. Efficient filtration depends on
adequate blood flow maintaining a consistent pressure through the glomerulus.
Many factors can alter this blood flow and pressure, includ-ing hypotension,
decreased oncotic pressure in the blood, and increased pressure in the renal
tubules from an obstruction.
Tubular reabsorption and tubular secretion:
The second and third steps of urine formation occur in the renal tubules and are
called tubular reabsorption and tubular secretion. In tubu-lar reabsorption, a
substance moves from the filtrate back into the peritubular capillaries or vasa
recta. In tubular secretion, a substance moves from the peritubular capillaries
or vasa recta into tubular filtrate. Of the 180 L (45 gallons) of filtrate that
the kidneys produce each day, 99% is reabsorbed into the bloodstream, resulting
in 1,000 to 1,500 mL of urine each day. Although most reabsorption occurs in
the proximal tubule, reabsorption occurs along the entire tubule. Reab-sorption
and secretion in the tubule frequently involve passive and active transport and
may require the use of energy. Filtrate becomes concentrated in the distal
tubule and collecting ducts under the influence of antidiuretic hormone (ADH) and be-comes urine, which then enters
the renal pelvis.
Excretion of Waste Products
The
kidney functions as the body’s main excretory organ, elimi-nating the body’s
metabolic waste products. The major waste product of protein metabolism is
urea, of which about 25 to 30 g is produced and excreted daily. All of this
urea must be excreted in the urine; otherwise it will accumulate in body
tissues. Other waste products of metabolism that must be excreted are
creatinine, phosphates, and sulfates. Uric acid, formed as a waste product of
purine metabolism, is also eliminated in the urine. The kidneys serve as the
primary mechanism for excreting drug metabolites.
Regulation of Electrolyte Excretion
When
the kidneys are functioning normally, the volume of elec-trolytes excreted per
day is exactly equal to the amount ingested. For example, the average American
daily diet contains 6 to 8 g each of sodium chloride (salt) and potassium
chloride. Nearly all of this is excreted in the urine. Electrolyte excretion
includes sodium and potassium.
SODIUM
More than 99% of the water and sodium
filtered at the glomeruli is reabsorbed into the blood by the time the urine
leaves the body. Water from the filtrate follows the reabsorbed sodium to
maintain osmotic balance. By regulating the amount of sodium (and there-fore
water) reabsorbed, the kidney can regulate the volume of body fluids. If more
sodium is excreted than ingested, dehydration re-sults; if less sodium is
excreted than ingested, fluid retention results.
The
regulation of sodium volume excreted depends on aldoste-rone, a hormone synthesized and released from the adrenal
cor-tex. With increased aldosterone in the blood, less sodium is excreted in
the urine because aldosterone fosters renal reabsorption of sodium. Release of
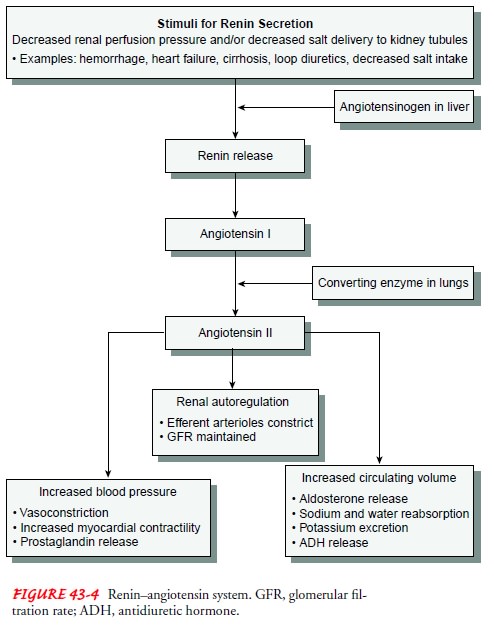
aldosterone from the adrenal cortex is largely under the control of angiotensin
II. Angiotensin II levels are in turn controlled by renin, an enzyme that is
released from specialized cells in the kidneys (Fig. 43-4). This complex system
is activated when pressure in the renal arterioles falls below normal levels,
as occurs with shock, dehydration, or decreased sodium chloride delivery to the
tubules. Activation of this system increases the retention of water and
expansion of intravascular fluid volume.
POTASSIUM
Potassium
is the most abundant intracellular ion, with about 98% of the total-body
potassium located intracellularly. To maintain a normal potassium balance in
the body, the kidneys are responsible for excreting more than 90% of the total
daily potas-sium intake. Several factors influence potassium loss through the
kidneys. Aldosterone causes the kidney to excrete potassium, in contrast to
aldosterone’s effects on sodium described previously. Acid–base balance, the amount
of dietary potassium intake, and the flow rate of the filtrate in the distal
tubule also influence the amount of potassium secreted into the urine.
Retention of potas-sium is the most life-threatening effect of renal failure.
Regulation of Acid Excretion
The
catabolism, or breakdown, of proteins results in the produc-tion of acid
compounds, in particular phosphoric and sulfuric acids. The normal daily diet
also includes a certain amount of acid materials. Unlike carbon dioxide (CO2), phosphoric and sulfuric
acids are nonvolatile and cannot be eliminated by the lungs. Be-cause
accumulation of these acids in the blood would lower its pH (making the blood
more acidic) and inhibit cell function, they must be excreted in the urine. A
person with normal kidney func-tion excretes about 70 mEq of acid each day. The
kidney is able to excrete some of this acid directly into the urine until the
urine pH reaches 4.5, which is 1,000 times more acidic than blood.
More
acid, however, usually needs to be eliminated from the body than can be
excreted directly as free acid in the urine. These excess acids are bound to
chemical buffers so they can be excreted in the urine. Two important chemical
buffers are phosphate ions and ammonia (NH3). When buffered with acid, ammonia be-comes
ammonium (NH4). Phosphate is present
in the glomeru-lar filtrate, and ammonia is produced by the cells of the renal
tubules and secreted into the tubular fluid. Through the buffer-ing process,
the kidney is able to excrete large quantities of acid in a bound form, without
further lowering the pH of the urine.
Regulation of Water Excretion
Regulation
of the amount of water excreted is also an important function of the kidney.
With high fluid intake, a large volume of di-lute urine is excreted. Conversely,
with a low fluid intake, a small volume of concentrated urine is excreted. A
person normally ingests about 1 to 2 L of water per day, and normally all but
400 to 500 mL of this fluid is excreted in the urine. The remainder is lost
from the skin, from the lungs during breathing, and in the feces.
OSMOLALITY
The degree of dilution or concentration of the urine can be mea-sured in terms of osmolality, the number of particles (electrolytes and
other molecules) dissolved per kilogram of urine. The filtrate in the
glomerular capillary normally has the same osmolality as the blood, with a
value of about 300 mOsm/L (300 mmol/L). As the filtrate passes through the
tubules and collecting ducts, the os-molality may vary from 50 to 1,200 mOsm/L,
reflecting the max-imal diluting and concentrating abilities of the kidney.
When a person is dehydrated or retaining fluid, less water is excreted, and
proportionately more particles are present in the urine, giving the urine a
concentrated appearance and a high osmolality. When a person excretes a large
volume of water, the particles are diluted. The urine appears dilute and the
osmolality is low. Certain sub-stances can alter the volume of water excreted
and are described as osmotically active. When these substances are filtered,
they pull water across the glomeruli and tubules and increase the volume of
urine. Glucose and proteins are two examples of osmotically active molecules.
Urine osmolality normally ranges from 300 to 1,100 mOsm/kg; however, after a
12-hour fluid restriction, that range narrows to 500 to 850 mOsm/kg. This wide
range of nor-mal makes the test valuable only when the kidneys’ concentrat-ing
and diluting abilities are questioned.
SPECIFIC GRAVITY
Specific gravity is a measurement of the kidney’s ability to
con-centrate urine. It compares the weight of urine (weight of particles) to
the weight of distilled water, which has a specific gravity of 1.000. Normal
urine specific gravity is 1.010 to 1.025 when fluid intake is normal. Factors
that may interfere with an accurate urine specific gravity reading include radiopaque
contrast agents, glucose, and proteins. Cold urine specimens may also produce a
falsely high reading. Several methods can be used to measure specific gravity:
· Multiple-test dipstick
(most common method), with a spe-cific reagent area for specific gravity
· Urinometer (least
accurate method), in which urine is placed in a small cylinder, and the
urinometer is floated in the urine; a specific gravity reading is obtained at
the meniscus level of the urine
· Refractometer, an
instrument used in a laboratory setting, which measures differences in the
speed of light passing through air and the urine sample
Urine
specific gravity depends largely on hydration status. When fluid intake
decreases, specific gravity normally increases. With high fluid intake,
specific gravity decreases. In patients with kidney disease, urine specific
gravity does not vary with fluid in-take, and the patient’s urine is said to
have a fixed specific grav-ity. Disorders or conditions that cause a low urine
specific gravity include diabetes insipidus, glomerulonephritis, and severe
renal damage. Those that can cause an increased specific gravity in-clude
diabetes mellitus, nephrosis, and excessive fluid loss.
ANTIDIURETIC HORMONE
ADH
(also known as vasopressin) regulates water excretion and urine concentration
in the tubule by varying the amount of water that is reabsorbed. ADH is a
hormone that is secreted by the posterior part of the pituitary gland in
response to changes in osmo-lality of the blood. With decreased water intake,
blood osmolal-ity tends to rise and stimulate ADH release. ADH then acts on the
kidney, increasing reabsorption of water and thereby return-ing the osmolality
of the blood to normal. With excess water in-take, the secretion of ADH by the
pituitary is suppressed; therefore, less water is reabsorbed by the kidney
tubule. This lat-ter situation leads to increased urine volume (diuresis).
A
dilute urine with a fixed specific gravity (about 1.010) or fixed osmolality
(about 300 mOsm/L) indicates an inability to concen-trate and dilute the urine,
a common early sign of kidney disease.
Autoregulation of Blood Pressure
Regulation
of blood pressure is also a function of the kidney. Spe-cialized vessels of the
kidney called the vasa recta constantly mon-itor blood pressure as blood begins
its passage into the kidney. When the vasa recta detect a decrease in blood
pressure, special-ized juxtaglomerular cells near the afferent arteriole,
distal tubule, and efferent arteriole secrete the hormone renin. Renin converts
angiotensinogen to angiotensin I, which is then converted to an-giotensin II,
the most powerful vasoconstrictor known. The vaso-constriction causes the blood
pressure to increase. The adrenal cortex secretes aldosterone in response to
stimulation by the pi-tuitary gland, which in turn is in response to poor
perfusion or increasing serum osmolality. The result is an increase in blood
pressure. When the vasa recta recognize the increase in blood pressure, renin
secretion stops. Failure of this feedback mecha-nism is one of the primary
causes of hypertension.
Renal Clearance
Renal clearance refers to the ability of the
kidneys to clear solutes from the plasma. A 24-hour collection of urine is the
primary test of renal clearance used to evaluate how well the kidney performs
this important excretory function. Clearance depends on several factors: how
quickly the substance is filtered across the glomeru-lus, how much of the substance
is reabsorbed along the tubules, and how much of the substance is secreted into
the tubules. It is possible to measure the renal clearance of any substance,
but the one measure that is particularly useful is the creatinine clearance.
Creatinine is an endogenous waste product of skeletal
musclethat is filtered at the glomerulus, passed through the tubules with
minimal change, and excreted in the urine. Hence, creatinine clear-ance is a
good measure of the glomerular
filtration rate (GFR). To calculate creatinine clearance, a 24-hour urine
specimen is col-lected. Midway through the collection, the serum creatinine
level is measured. The following formula is then used to calculate the
creatinine clearance:
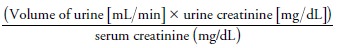
The
normal adult GFR is about 100 to 120 mL/min (1.67 to 2.0 mL/sec). Creatinine
clearance is an excellent measure of renal function; as renal function
declines, creatinine clearance decreases.
Regulation of Red Blood Cell Production
When
the kidneys sense a decrease in the oxygen tension in renal blood flow, they
release erythropoietin. Erythropoietin stimulates the bone marrow to produce
red blood cells (RBCs), thereby in-creasing the amount of hemoglobin available
to carry oxygen.
Vitamin D Synthesis
The
kidneys are also responsible for the final conversion of in-active vitamin D to
its active form, 1,25-dihydroxycholecalciferol. Vitamin D is necessary for
maintaining normal calcium balance in the body.
Secretion of Prostaglandins
The
kidneys also produce prostaglandin E (PGE) and prostacy-clin (PGI), which have
a vasodilatory effect and are important in maintaining renal blood flow.
Urine Storage
The
bladder is the reservoir for urine. Both bladder filling and emptying are
mediated by coordinated sympathetic and parasym-pathetic nervous system control
mechanisms involving the de-trusor muscle and the bladder outlet. In an infant,
bladder filling and emptying are mediated within the micturition center in the
pons area of the brain stem. By the time a child is 3 to 4 years old, the
cerebral cortex is mature enough to cause a conscious aware-ness of bladder
filling. This conscious awareness of bladder filling occurs as a result of
sympathetic neuronal pathways that travel via the spinal cord to the level of
T10-12, where peripheral, hypo-gastric nerve innervation allows for continued
bladder filling. As bladder filling continues, stretch receptors in the bladder
wall are activated, coupled with the desire to void. This information from the
detrusor muscle is relayed back to the cerebral cortex via the parasympathetic
pelvic nerves at the level of S2 through S4. Normally, the pressure in the
bladder remains low even as the urine accumulates, due to the bladder’s
compliance, or ability to expand with increasing urine volumes (Appell, 1999).
Bladder
compliance is due in part to the smooth muscle lin-ing of the bladder and
collagen deposits within the wall of the bladder, as well as to neuronal
mechanisms that inhibit the de-trusor muscle from contracting (specifically,
adrenergic receptors that mediate relaxation). To maintain adequate kidney
filtration rates, bladder pressure during filling must remain lower than 40 cm
H2O. Ordinarily, the first
sensation of bladder filling occurs when there is approximately 100 to 150 mL
of urine in the blad-der. The first sensation of bladder fullness is
transmitted to the central nervous system when the bladder has reached
approxi-mately half of its capacity, about 200 to 300 mL in adults, and an
initial desire to void occurs. A marked sense of fullness with a strong desire
to void usually occurs when the bladder contains 350 mL or more of urine
(“functional capacity”). During anes-thesia, the average adult bladder under
pressure of 60 cm H2O can hold 1,500 to 2,000 mL (“anatomic capacity”).
During nor-mal circumstances, with appropriate bladder wall innervation,
capacity would never reach this level because of the tremendous pain and
pressure that such fullness would cause. Neurologic changes to the bladder at
the level of the supraspinal, spinal, or bladder wall itself can cause
abnormally high volumes of urine to be stored due to a decreased or absent urge
to void. Under nor-mal circumstances with average fluid intake of approximately
1,500 to 2,000 mL per day, the bladder should be able to store urine for
periods of 2 to 4 hours at a time during the day. At night, the release of
vasopressin in response to decreased fluid in-take causes less production of
urine that is more concentrated. This phenomenon usually allows the bladder to
continue filling for periods of 6 to 8 hours in adolescents and adults. In
older in-dividuals, decreasing bladder compliance and vasopressin levels cause
nighttime bladder filling to decrease to periods ranging from 3 to 6 hours
(Appell, 1999).
Bladder Emptying
Micturition
(voiding) normally occurs approximately eight times in a 24-hour period. It is
activated via the micturition reflex arc within the sympathetic and
parasympathetic nervous system, which causes a coordinated sequence of events.
Initiation of voiding occurs when the efferent pelvic nerve, which originates
in S2 to S4, stimulates the bladder to contract, resulting in complete
relaxation of the striated urethral sphincter and followed by a fall in
urethral pressure, contraction of the detrusor muscle, opening of the vesicle
neck and proximal urethra, and flow of urine. This coordinated effort by the
parasympathetic system is mediated by muscarinic and, to a lesser extent,
cholinergic receptors within the detrusor muscle. The pressure generated in the
bladder during micturition is about 20 to 40 cm H2O in females. It is somewhat
higher and more variable in males ages 45 and older due to the gland that
surround the proximal urethra. An obstruction of the bladder outlet, such as in
advanced benign prostatic hyperplasia (BPH), results in abnormally high voiding
pressure with a slow, prolonged flow of urine. In females, gravity drains any
urine remaining in the urethra; in males, voluntary muscle contractions expel
the urine (Wein, 2001).
If the
spinal pathways from the brain to the urinary system are destroyed (eg, after a
spinal cord injury), reflex contraction of the bladder is maintained, but
voluntary control over the process is lost. In both situations, the detrusor
muscle can contract and expel urine, but the contractions are generally
insufficient to empty the bladder completely, so residual urine (urine left in
the bladder after voiding) remains. Normally, residual urine amounts to no more
than 50 mL in the middle-aged adult and less than 50 to 100 mL in the older
adult. Chronic urine retention is more prevalent in older men and women (Gray,
2000b).
Related Topics