Organisation of Life | Chapter 18 | 8th Science - Physiological Processes | 8th Science : Chapter 18 : Organisation of Life
Chapter: 8th Science : Chapter 18 : Organisation of Life
Physiological Processes
Physiological
Processes
The ways in which biomolecules,
cells, tissues, organs and organs systems work together to accomplish the
complex goal of sustaining life are called physiological processes. Let us
study about some of them here.
Homeostasis
Homeostasis is a property of human
biological system where the self-regulating
process tends to maintain the balance for the survival. The regulation takes
place in a defined internal environment. Mammals are capable of maintaining
constant body temperature despite the changes in the external temperature.
Behavioural and physiological responses are the two important regulating
mechanisms that maintain the stability of homeostasis.
In simple terms, it could be
referred as a balance in a system to maintain a stable internal environment for
the survival of the animal. If the homeostasis regulates successfully, life
continues or if unsuccessful, death or disaster occurs.
All the processes of integration and
co-ordination of function are mediated by nervous and hormonal system. The
liver, kidney, and brain (hypothalamus), autonomic nervous system and the
endocrine system help to maintain homeostasis.
Maintenance of body fluid
concentrations, body temperature are done by various bio-physical and
bio-chemical methods. Human beings are warm blooded in nature i.e, they
maintain their body temperature as constant. When the body temperature raises
sweat is produced to bring the temperature down. When the body temperature
lowers heat is produced by the muscular work by shivering. This is an example
for homeostasis.
The control of blood glucose level
is another example in which insulin hormone is secreted whenever the blood
glucose level raises and glucagon hormone is secreted whenever the blood
glucose level reduces.
Diffusion
Diffusion is the movement of
particles from an area of higher
concentration to lower concentration.
The overall effect is to equalize concentration throughout the medium.
Examples for diffusion include,
perfume filling a whole room and the movement of small molecules across a cell
membrane. One of the simplest demonstrations of diffusion is adding a drop of
ink to water.
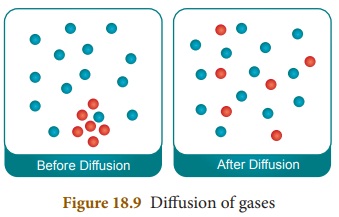
What will happen when an incense
stick is lit up in a room? How do we feel? The fragrance spreads over the
entire room. The movement of molecules or ions is from a region of higher
concentration to region of lower concentration. You can smell incense stick
after lighting because the smoke diffuses in the air and makes its way to your
nose. Let us think of the following. How does the smell spread in the entire
room? Does the smell spread uniformly in the entire room? Can you give any
other examples?
There are other processes in which
substances move in liquid medium. For an example when a tea pack is immersed in
a cup of hot water the tea powder particles disperse in to water by diffusion.

The mixing of
foodstuffs and digestive juices in the gut occurs by diffusion. Exchange of
respiratory gases (Oxygen and Carbondioxide) between blood and tissue fluids
between tissue fluid and cells also occurs by diffusion.
Osmosis
Osmosis is the movement of solvent
particles across a semipermeable membrane from a dilute solution into a
concentrated solution. The solvent moves to dilute the concentrated solution
and equalize the concentration on both sides of the membrane.
The movement of liquids in and out
of cells is dependent on the concentration of the solution surrounding it.
There are three types of situations in which this could vary.
Isotonic
Here the concentration of external
and internal solution of the organism are the same.
Hypotonic
Here the external solution
concentration is less compared to the concentration of the inner solution of an
organism. In this case water will rush into the organism.
Hypertonic
Here the external solution
concentration is greater than the concentration of the inner solution of an
organism. In this case the water will rush out of the organism.
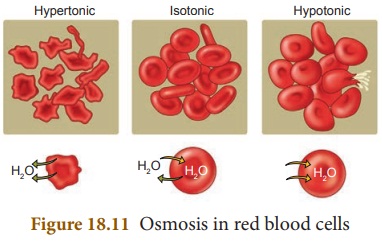
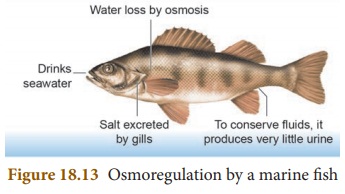
Osmoregulation
The term osmoregulation was coined
by Hober in 1902. Osmoregulation is
the process by which an organism regulates the water balance in its body and
maintains the homeostasis of the body. It includes controlling excess water
loss or gain and maintaining the fluid balance and the osmotic concentration,
that is, the concentration of electrolytes. It ensures that the fluids in the
body do not get too diluted or concentrated.
Organisms are divided into two types
based on osmoregulation. They are Osmoconformers and Osmoregulator.
Osmoconformers
These organisms try to maintain the
osmolality of their body matching with their surroundings. Most of the
invertebrates, marine organisms are osmoconformers.
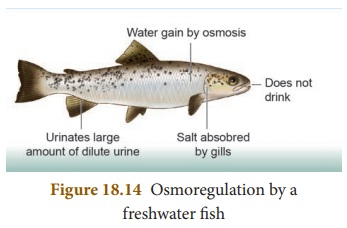
Osmoregulators
These organisms maintain their
internal osmolality, which can be extremely different from that of the
surrounding environment, through physiological processes
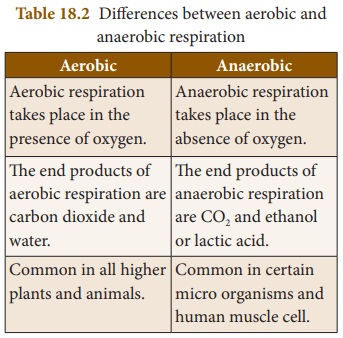
Cellular respiration
Cellular respiration is the process
by which organisms break down glucose into a form that the cell can use as
energy. This energy is then made available to living cells in the form of ATP. Cellular respiration takes place
in the cytoplasm and mitochondria of the cells. The cellular respiration is
classified into two types: aerobic
respiration and anaerobic respiration.
a.
Aerobic respiration
In this type of respiration, the
food substances are completely oxidized into H2O and CO2
with the release of energy. It requires atmospheric oxygen and all higher
organisms respire aerobically. This reaction releases a large amount of energy.
Glucose + Oxygen → Carbon dioxide +
Water + Energy
b.
Anaerobic respiration
In this type of respiration, partial
oxidation of food takes place and the organisms release energy in the absence
of oxygen. This type of respiration occurs in organisms like yeast. Ethyl alcohol
or lactic acid and carbon dioxide are the by-products of this process. This
reaction releases very little energy because glucose is not completely oxidized.
For example, yeast cells convert
glucose into carbon dioxide and ethanol, with the release of energy, without
using oxygen.
Glucose → Ethanol + Carbon dioxide +
Energy
Metabolism
Metabolism is the sum of chemical
reactions by which living organisms sustain their life. Metabolism consists of
anabolism (the buildup of substances) and catabolism (the breakdown of
substances). The term metabolism is commonly used to refer specifically to the
breakdown of food and its transformation into energy, cellular products and
waste elimination.
More to know
Aerobic respiration
releases 19 times more energy than anaerobic respiration from the same amount
of glucose. In aerobic respiration each glucose molecules produce 36 ATPs.
a.
Anabolism
Anabolism or constructive
metabolism, is all about building and storing. It supports the growth of new
cells, the maintenance of body tissues, and the storage of energy for use in
the future. During anabolism, small molecules are changed into larger, more
complex molecules of carbohydrate, protein, and fat.
Example
Glucose → Glycogen and other sugars
Amino acids → Enzymes, hormones,
proteins
Fatty acids → Cholesterol and other
steroids
b.
Catabolism
Catabolism or destructive
metabolism, is the process that produces the energy required for all activity
in the cells. In this process, cells break down large molecules (mostly
carbohydrates and fats) to release energy. This energy release provides fuel
for anabolism, heats the body, and enables the muscles to contract and the body
to move. As complex chemical units are broken down into more simple substances,
the waste products released in the process of catabolism are removed from the
body through the skin, kidneys, lungs, and intestines.
Example
Carbohydrates → Glucose
Glucose → CO2 , Water and
Heat
Protein → Amino acid
The repeated anabolism and
catabolism reactions maintain the homeostatic condition in the organism. The
metabolic process is the cause for maintaining ionic balance in the body. It is
also responsible for movement, growth, development, maintenance and repair of
the cells, tissues and the human body. These metabolic reactions occur in
different organs of living species.
More to know
Basal metabolism
refers to the minimum energy required to maintain the normal activities of the
body during complete rest in a warm atmosphere, 12 – 18 hours after the intake
of food.
Related Topics