Chapter: 11th Geography : Chapter 6 : Atmosphere
Ozone and Ozone Depletion
Ozone
and Ozone Depletion
Ozone (O3) is form of oxygen that
combines three atoms into each molecule. It absorbs and filters the harmful
ultraviolet B radiation coming from the sun. This way the ozone layer protects
all life on earth. However, ozone is harmful when it develops near the ground.
It causes health problems like asthma and other respiratory illness.
Ozone
Depletion: A steady decline in the
concentration of ozone in the
earth’s stratosphere (the ozone layer)
is called ozone depletion.
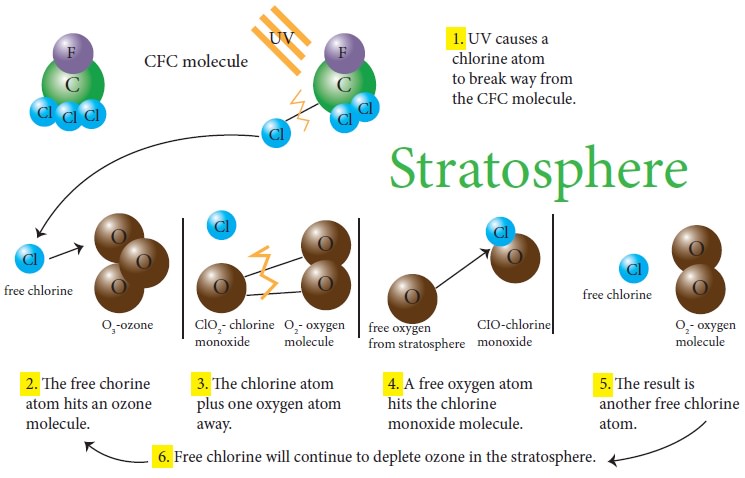
Ozone depletion occurs when chloro fluoro carbon (CFC) and halon gases, formerly found in aerosol spray cans and refrigerants are released into the atmosphere and they cause chemical reactions that break down ozone molecules and reduce the concentration of them. Nitrogen oxide released by emitted by supersonic aircrafts can also destroy the ozone molecules to break down. Ozone-depleting substances are present throughout the stratospheric ozone layer because they are transported great distances by atmospheric air motions. The severe depletion of the Antarctic ozone layer known as the “ozone hole” occurs because of the special atmospheric and chemical conditions that exist there and nowhere else on the globe. The very low winter temperatures in the Antarctic stratosphere cause polar stratospheric clouds (PSCs) to form. Special reactions that occur on PSCs, combined with the relative isolation of polar stratospheric air, allow chlorine and bromine reactions to produce the ozone hole in Antarctic springtime.
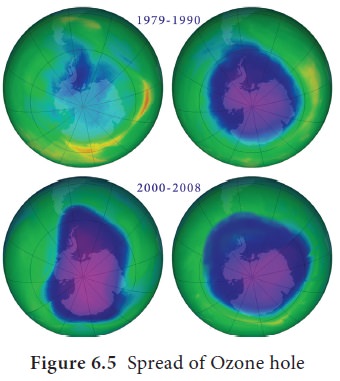
Satellite images of the earth over
last decades observed that the atmospheric ozone layer is getting thinner. On
October 2, 2015, the ozone hole was recorded to its maximum size of 28.2
million sq.km over Antarctica
(Figure 6.5). The size of the ozone hole is larger than the size of continent
of North America. The ozone holes over Antarctica allow the ultraviolet
radiation to enter and cause global warming, skin cancer, eye cataract and even
blindness.
Depletion
of the ozone layer
has consequences on human, animal, plants
and micro organisms. This typically results from higher UV levels reaching us
on earth. Research confirms that high levels of UV rays cause non-melanoma skin
cancer.
To protect the ozone layer for our
future generation, avoid using products which are emitting pollutants such as
aerosol sprays, blowing agents for foams and packing materials, as solvents and
as refrigerants.
Related Topics