Chapter: Modern Medical Toxicology: Corrosive(Caustic) Poisons: Organic Acids
Oxalic Acid - Corrosive(Caustic) Poisons
Oxalic Acid
Synonyms
Ethanediolic
acid; Dicarboxylic acid; Salt of sorrel.
Physical Appearance/Derivatives
·
Oxalic acid, the simplest
dicarboxylic acid, is a potentially naturally present as a salt in many plants.
Oxalic acid is a relatively strong acid, and forms a white, dihydrate
precipitate.
·
Oxalic acid occurs naturally in
plants and vegetables such as wood sorrel (Fig
6.5), rhubarb (Fig 6.6), and
spinach (Fig 6.7). Alkali extraction
of sawdust and the metabolism of many moulds will also produce oxalic acid.



·
Rust and ink stain removers, and
ceramics. It is also used in general metal and equipment cleaning, wood
cleaning, process engraving, printing and dyeing, bleaching, textile finishing,
leather tanning, and photography.
Usual Fatal Dose
About
15 to 30 grams of oxalic acid.
Mode of Action
Liquid
oxalic acid has moderate corrosive action on skin and mucosa. Systemic
absorption leads to hypocalcaemia, since it reacts with calcium in plasma, and
insoluble calcium oxalate is precipitated which accumulates in the liver,
kidneys, heart, lungs, and blood, and is excreted in the urine.
Clinical Features
Local:
Whitish
or yellowish corrosion (mucosa), or discolouration (skin), with underlying
congestion. The corroded mucosa is ![]() often referred to as “scalded” in
appearance. Production of acid haematin however can turn the mucosa blackish.
often referred to as “scalded” in
appearance. Production of acid haematin however can turn the mucosa blackish.
Systemic :
Vomiting
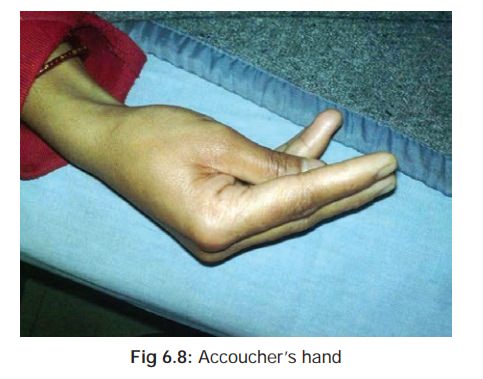
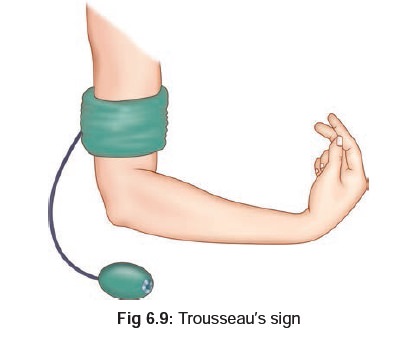
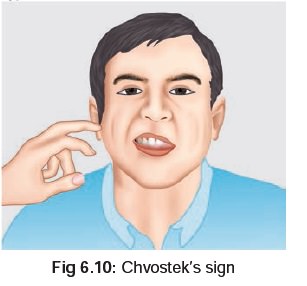
and diarrhoea, followed by signs and symptoms of hypocalcaemia (tetany), characterised by tonic muscle
spasms, cramping, and accoucher’s hand*
(Fig
6.8). There is often a positive Trousseau’s**(Fig
6.9), and Chvostek’s*** sign (Fig6.10).
Pupils are usually dilated. Later there may be metabolicacidosis, ventricular
fibrillation, and renal failure. Calcium oxalate crystals can be deposited in
the liver resulting in hepatic necrosis and failure in severe cases. Milder
cases may manifest as elevated serum liver enzymes.
Diagnosis
· Demonstration of urinary oxalate
crystals (Fig 6.11) which may occur
either as monohydrates (prism or needle-like), or dihydrates (tent or envelope
shaped).
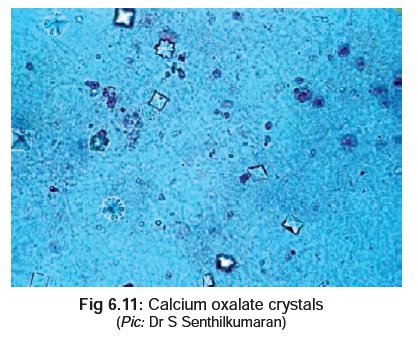
· Oxalic acid can be measured in the
urine by colourimetry. The normal upper limit is 40 to 50 mg/24 hours.
· Average serum oxalate concentration
is said to be 1.4 mg/L.
Treatment
· Stomach wash with calcium gluconate
or lactate solution.
· Calcium gluconate IV (10 ml, 10%
solution). Dialysis or exchange transfusion for renal failure.
· Affected skin or eye should be
washed copiously with water.
Autopsy Features
·
“Scalded” mucosa of GI tract
(especially stomach). Sometimes there is a brown or black colour due to acid
haematin. Crystals of calcium oxalate may be demonstrated in scrapings of the
mucosa, (examine with polarising mico-scope).
·
Whitish or yellowish discolouration
of corroded areas.
·
Microscopic evidence of renal
damage.
Forensic Issues
·
Most cases of oxalic acid or oxalate
poisoning result from accidental causes, e.g. mistaking the substance for Epsom
salt, sodium bicarbonate, etc. Accidental poisoning may also result from
excessive ingestion of certain vegetables rich in oxalates (rhubarb leaves,*
sorrel, etc.). Even tea is said to contain significant amounts of oxalate.
·
Chronic consumption of oxalic acid can
lead to renal calculi with consequent renal colic.
·
Occasional cases of suicide and
homicide with oxalic acid have been reported.
Related Topics