Chapter: Modern Medical Toxicology: Corrosive(Caustic) Poisons: Organic Acids
Carbolic Acid - Corrosive(Caustic) Poisons

Carbolic Acid
Synonyms
Hydroxybenzene;
Phenol; Benzenol; Phenyl alcohol.
Physical Appearance
·
Colourless, needle-like crystals
which turn pink and liquefy when exposed to air. It is a coal tar derivative
(creosote).
·
Commercial phenol is a brownish
liquid containing impurities like cresol (Fig
6.2). Household phenol (often sold as phenyle) contains 5% phenol in water.
It has a characteristic, aromatic odour (“hospital odour”).

Derivatives:
·
Catechol, cresols, menthol,
resorcinol, thymol: Toxic
·
Hexyl resorcinol, naphthol: Less Toxic
·
Tannic acid: Least Toxic.
Uses
Carbolic acid was introduced as a
disinfectant in the 19th century by Lemaire, and quickly became popular
ever since Lord Lister (Fig 6.3) advocated its use in surgery
for asepsis. Even today it is popular as a hospital and household disinfectant
along with its related counterpart Lysol,*
even though several safer and more effective alternatives have been developed
including cetrimide, chlorhexidine (Savlon),
chloroxylenol, parachlorometaxylenol (Dettol),
terpineol, and xylenol. The various uses of carbolic acid are as follows

·
Antiseptic
and disinfectant: especially for sterilising
floors,walls, furnishings, glassware, and instruments.
·
Preservative:
Phenol is a commonly used preservative ininjectable
medications, e.g. glucagon, pethidine, neostig-mine, quinidine, and
epinephrine.
·
Pharmaceuticals
·
Medical
uses:
o “Face
peel” in plastic surgery.
o Neurolysis
for spasticity (by injecting phenol solution into neuromuscular junctions).
o Phenol
is still used in preparations for treatment of
localised skin disorders (Castellani’s paint), and as a local anaesthetic.
Usual Fatal Dose
·
Probable oral lethal dose is
reported at 50 to 500 mcg/kg.
·
Ingestion of 1 gram of phenol has
caused death. 25 to 50 ml of household phenol can cause death. Fatalities
·
have been reported even with much
less quantities. The UFD for Lysol is 60 to 120 ml.
Toxicokinetics
Carbolic
acid is rapidly absorbed through GI mucosa, lungs, and even intact skin.
Dilution may actually increase absorption and enhance toxicity.
Mode of Action
Carbolic
acid is actually a very mild corrosive, but has profound systemic effects after
absorption. There is CNS depression, metabolic acidosis, and renal damage.
Clinical Features
Acute Poisoning
Local: Skin or mucosal contact results in mild corrosion with
hardening and whitish discolouration. However the white eschar (especially in
the skin) drops off in a few days, leaving a brown stain. Locally there may be
burning pain followed by tingling, numbness, and anaesthesia.
Systemic:
·
GIT—Burning pain, vomiting.
·
CNS—Vertigo, convulsions, coma. Pupils are constricted.
·
RS—Tachypnoea, bronchospasm, pulmonary oedema.
·
CVS—Tachycardia, hypotension, cardiac arrhythmias ![]() Blood—Haemolysis, methaemoglobinaemia.
Blood—Haemolysis, methaemoglobinaemia.
·
Metabolic—Hypothermia, with cold and clammy skin, metabolic
acidosis.
·
Hepatorenal—Oliguria, with scanty urine which turns greenish
or brownish on exposure to air because of phenolic metabolites (hydroquinone
and pyrocatechol). Later there is renal and hepatic failure.
·
Deaths have occurred even after skin contact with carbolic
acid. Table 6.2 lists the agents
which can cause serious poisoning through dermal absorption.

Chronic Poisoning (Phenol Marasmus)
This
was common in earlier days among medical personnel when phenol was routinely
used as a skin disinfectant. It is characterised by anorexia, weight loss,
headache, vertigo, dark urine, and pigmentation of skin and sclerae (ochronosis).
Diagnosis
·
Typical odour in the vicinity of the
patient.
· Urine collected and stored in a
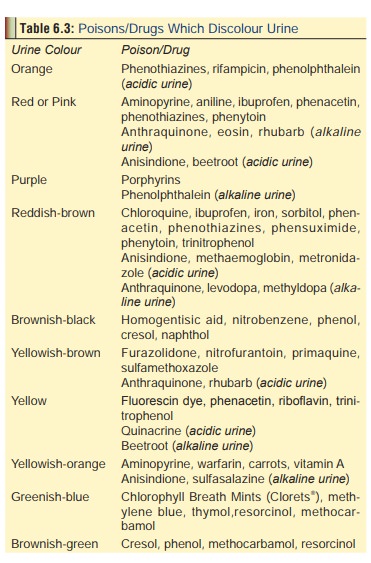
transparent container shows a gradual change in colour to brown or green (Table 6.3 contains a list of poisons
and drugs which discolour urine).
· To 10 ml of urine, add 1 ml of 10%
ferric chloride. A purple or blue colour which persists even on heating
indicates phenol poisoning. Cresol is associated with green colour.
· When 10 ml of urine is boiled with
Millon’s reagent,* a red colour is produced.
Treatment
· Decontaminate skin by copious
washing.
· Stomach wash can be done preferably
with sodium or magnesium sulfate solution.
· Activated charcoal in the usual
manner.
· Treatment of methaemoglobinaemia
(with methylene blue).
· Convulsions can be managed with
benzodiazepines or barbiturates.
· Supportive measures.
Autopsy Features
·
Distinct odour of phenol, especially
around the mouth and in the stomach contents.
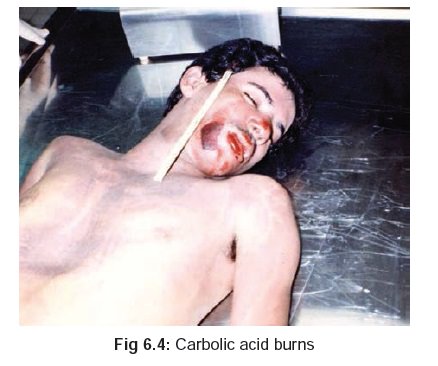
·
Corroded areas are at first white,
but if death has been delayed they turn brownish (Fig 6.4).
Gastric
mucosa is greyish white, swollen, and hardened (leathery), but Lysol poisoning
is asociated with soapy and soft mucosa.
·
Urine is greenish or brownish.
Forensic Issues
Most
cases of poisoning are accidental in nature arising out of occupational
exposure or therapeutic misuse. Formerly, suicidal ingestions were common.
Poisoning with phenolic derivatives causes similar but usually less severe
manifestations, and must be treated on the same lines as phenol.
Related Topics