Chapter: Modern Medical Toxicology: Corrosive(Caustic) Poisons: Organic Acids
Acetic Acid - Corrosive(Caustic) Poisons

Organic Acids
Organic
acids differ from inorganic acids in two major respects:
·
They are weaker in action locally.
·
They are better absorbed into the
systemic circulation, and therefore have more powerful remote action.
Acetic Acid
Synonyms
Ethanoic
acid; Ethylic acid; Methane carboxylic acid; Pyroligenous acid.
Physical Appearance
Colourless,
volatile liquid with a characteristic pungent odour. At 100C
to 150C, the acid occurs in crystalline
form (glacial acetic acid).
Uses
·
60% solution: printing, dyeing,
plastics, and rayon manu-facturing, hat making.
·
6 to 40% solution: disinfectant,
pharmaceuticals, hair wave neutraliser.
·
4 to 5% solution: vinegar (Fig 6.1).

Usual Fatal Dose
About
50 to 100 ml of concentrate acetic acid.
Mode of Action
In
concentrated form, acetic acid is a corrosive (albeit mild), while in dilute
form it acts as an irritant. Systemic absorption leads to haemolysis,
haemoglobinuria, and renal failure.
Clinical Features
·
Local
effects: Mild grey-brown corrosion with concen-trated acid. Chronic
exposure to fumes causes darkening of skin.
·
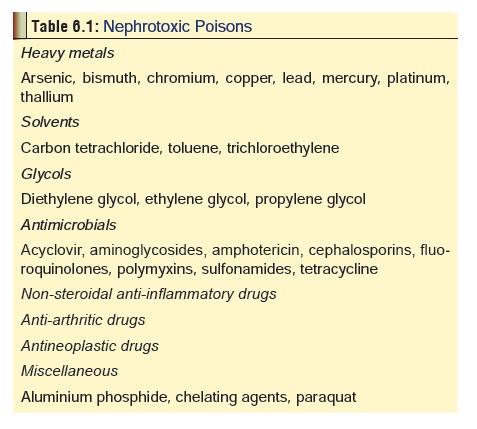
Ingestion:
Pain, haematemesis, haemolysis, diarrhoea,disseminated intravascular
coagulation, and renal failure (Table
6.1 lists some common nephrotoxic poisons).
·
Inhalation:
Bronchopneumonia, pulmonary oedema.Chronic exposure leads to erosion of front
teeth, chronic rhinitis, pharyngitis, and bronchitis.
Diagnosis
·
Odour of vinegar in the vicinity of
the patient.
·
When a small quantity of stomach
contents is gently heated with ethyl alcohol and 1 drop of sulfuric acid, there
is emanation of a fruity odour.
Treatment
This
is on general lines, with special attention paid to correction of acidosis and
renal damage. Stomach wash can be done with caution if the patient is seen
early.
Autopsy Features
· Odour of vinegar around the mouth
and in the gastric contents.
· The affected areas of skin and
mucosa may be greyish brown. Viscera meant for chemical analysis must not be
preserved with rectified spirit. Saturated saline should be used instead.
Forensic Issues
·
Most cases of poisoning are
accidental. There are however occasional reported cases of suicide.
It
is pertinent to mention here that if paraldehyde meant for therapeutic use is stored
improperly (e.g. in badly stoppered, transparent glass containers), it degrades
into acetic acid with resultant iatrogenic poisoning when administered to a
patient.
Related Topics