Types, Experiment, Plasmolysis - Osmosis | 11th Botany : Chapter 11 : Transport in Plants
Chapter: 11th Botany : Chapter 11 : Transport in Plants
Osmosis
Osmosis
Osmosis
(Latin: Osmos-impulse, urge) is a special type of diffusion. It represents the movement of water or
solvent molecules through a selectively permeable membrane from the place of its higher concentration (high water potential) to the
place of its lower concentration (low water potential).
Types of
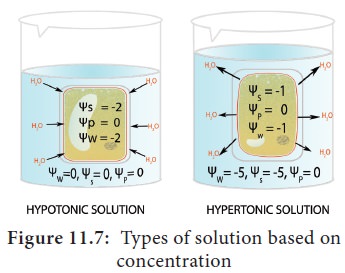
Solutions based on concentration
i.
Hypertonic (Hyper = High; tonic
= solute): This is a strong solution (low solvent/ high solute /
low Ψ) which attracts solvent from other solutions.
ii.
Hypotonic (Hypo = low; tonic
= solute):Â This is a weak solution (high solvent /low or zero solute /
high Ψ) and it diffuses water out to other solutions (Figure 11.7).
iii. Isotonic (Iso =
identical; tonic = soute): It refers to two
solutions having same concentration. In this condition the net movement of
water molecule will be zero.
The term hyper, hypo and isotonic are relative terms which can be used only in comparison with another solution.
Thistle funnel experiment
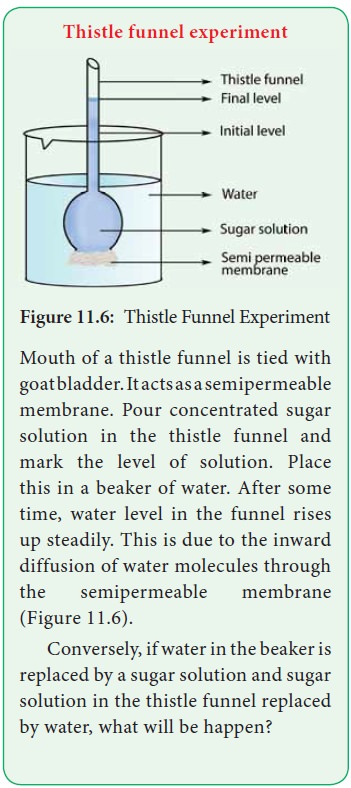
Mouth of a thistle funnel is tied with goatbladder.Itactsasasemipermeable membrane. Pour concentrated sugar solution in the thistle funnel and mark the level of solution. Place this in a beaker of water. After some time, water level in the funnel rises up steadily. This is due to the inward diffusion of water molecules through the semipermeable membrane (Figure 11.6).
Conversely, if water in the beaker is replaced by a sugar
solution and sugar solution in the thistle funnel replaced by water, what will
be happen?
1. Types of osmosis
Based on
the direction of movement of water or solvent in an osmotic system, two types
of osmosis can occur, they are Endosmosis and Exosmosis.
i.
Endosmosis: Endosmosis is defined as the
osmotic entry of solvent into a cell or a system when it is placed in a pure
water or hypotonic solution.
For
example, dry raisins (high solute and low solvent) placed in the water, it
swells up due to turgidity.
ii.
Exosmosis: Exosmosis is defined as the
osmotic withdrawal of water from a cell or system when it is placed in a
hypertonic solution. Exosmosis in a plant cell leads to plasmolysis.
2.
Plasmolysis
(Plasma = cytoplasm; lysis = breakdown)
When a
plant cell is kept in a hypertonic solution, water leaves the cell due to exosmosis. As a result of water loss, protoplasm shrinks and the cell
membrane is pulled away from the cell wall and finally, the cell becomes flaccid. This process is named as plasmolysis.
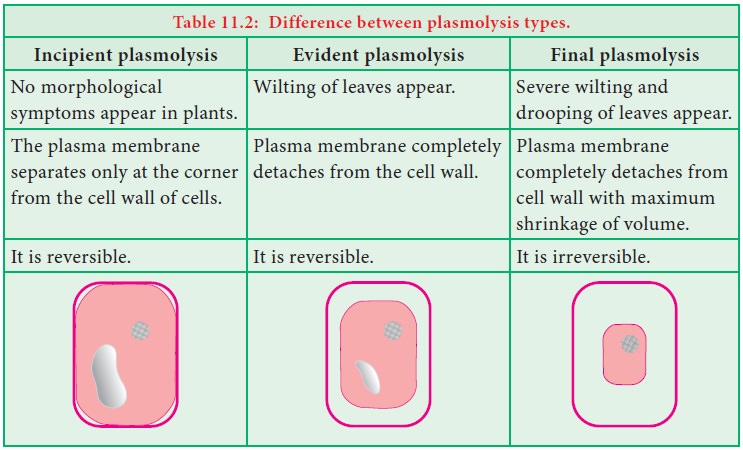
Wilting
of plants noticed under the condition of water scarcity is an indication of
plasmolysis. Three types of plasmolysis occur in plants: i) Incipient
plasmolysis
ii)
Evident plasmolysis and iii)
Final plasmolysis. Differences among them are given in table 11.2.
Significance
Plasmolysis
is exhibited only by living cells and so it is used to test whether the cell is
living or dead.
3. Deplasmolysis
The
effect of plasmolysis can be reversed, by transferring them back into water or hypotonic solution. Due to endosmosis, the cell becomes turgid again. It
regains its original shape and size. This phenomenon of the revival of the
plasmolysed cell is called deplasmolysis.
Example: Immersion of dry raisin in water.
Potato Osmoscope
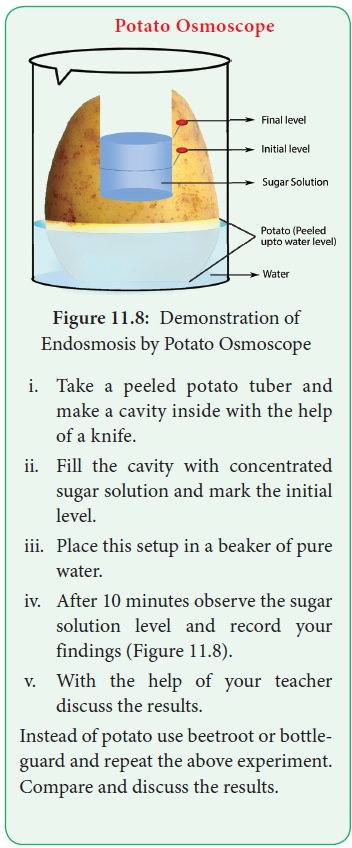
i. Take a peeled potato tuber and make a cavity inside with the help of a knife.
ii. Fill the cavity with concentrated sugar solution and mark the initial level.
iii. Place this setup in a beaker of pure water.
iv. After 10 minutes observe the sugar solution level and record your findings (Figure 11.8).
v. With the help of your teacher discuss the results.
Instead of potato use beetroot or bottle-guard and repeat the
above experiment. Compare and discuss the results.
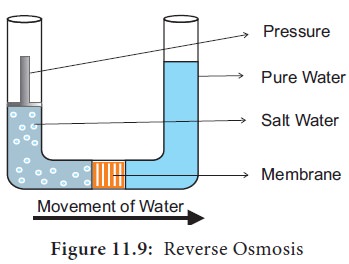
4. Reverse Osmosis
Reverse
Osmosis follows the same principles of osmosis, but in the reverse direction.
In this process movement of water is reversed by applying pressure to force the
water against a concentration gradient of the solution. In regular osmosis, the
water molecules move from the higher concentration (pure water
hypotonic)
to lower concentration (salt water = hypertonic). But in reverse osmosis, the
water molecules move from the lower concentration (salt water = hypertonic) to
higher concentration (pure water = hypotonic) through a selectively permeable
membrane (Figure 11.9).
Uses: Reverse osmosis is used for purification of drinking water and
desalination of seawater.
Related Topics