Chapter: 11th Botany : Chapter 11 : Transport in Plants
Mineral Absorption in Plants
Mineral Absorption
Minerals
in soil exist in two forms, either dissolved in soil solution or adsorbed by
colloidal clay particle. Previously, it was mistakenly assumed that absorption
of mineral salts from soil took place along with absorption of water. But
absorption of minerals and ascent of sap are identified as two independent
processes. Minerals are absorbed not only by root hairs but also by the cells
of epiblema.
Plasma
membrane of root cells are not permeable to all ions and also all ions of same
salt are not absorbed in equal rate.
Penetration
and accumulation of ions into living cells or tissues from surrounding medium
by crossing membrane is called mineral
absorption. Movement of ions into
and out of cells or tissues is termed as transport or flux. Entry of the ion into cell is called influx and exit is called efflux.
Various theories have been put forward
to explain this mechanism. They are categorized under passive mechanisms (without
the involvement of metabolic energy) and active mechanisms (involvement of
metabolic energy).
1. Passive Absorption
1. Ion-Exchange:
Ions of
external soil solution were exchanged with same charged (anion for anion or
cation for cation) ions of the root
cells. There are two theories explaining this process of ion exchange namely:
i. 
Contact exchange and ii.  Carbonic acid exchange.
i.
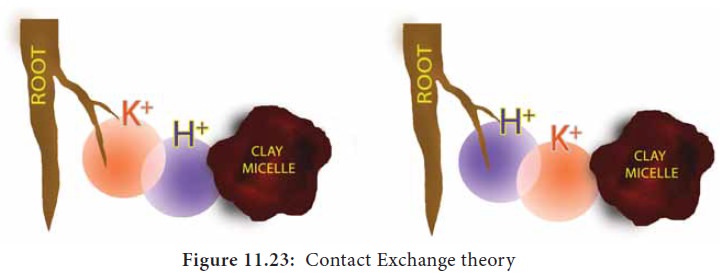
Contact Exchange Theory:
According
to this theory, the ions adsorbed on the surface of root cells and clay
particles (or clay micelles) are not held tightly but oscillate within a small
volume of space called oscillation
volume. Due to small space, both ions overlap each other’s oscillation
volume and exchange takes place (Figure 11.23).
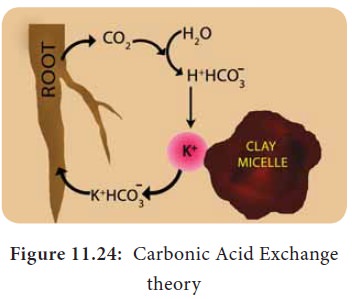
ii. Carbonic Acid Exchange Theory:
According
to this theory, soil solution plays an important role by acting as a medium for
ion exchange. The CO2 released during respiration of root cells
combines with water to form carbonic acid (H2CO3).
Carbonic acid dissociates into H+ and HCO3– in the soil solution.
These H+ ions exchange with cations adsorbed on clay particles and the cations
from micelles get released into soil solution and gets adsorbed on root cells
(Figure 11.24).
2. Active Absorption
Absorption
of ions against the concentration gradient with the expenditure of metabolic
energy is called active absorption.
In plants, the vacuolar sap shows
accumulation of anions and cations against the concentration gradient which
cannot be explained by theories of passive absorption. Mechanism of active
absorption of salts can be explained through Carrier concept.
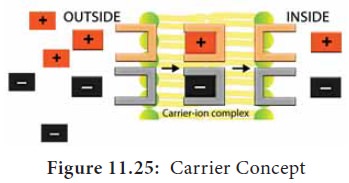
Carrier Concept:
This
concept was proposed by Van den Honert in 1937. The cell membrane is largely impermeable to free ions.
However, the presence of carrier
molecules in the membrane acts as a vehicle to pick up or bind with ions to
form carrier-ion-complex, which
moves across the membrane. On the inner surface of the membrane, this complex
breaks apart releasing ions into cell while carrier goes back to the outer
surface to pick up fresh ions (Figure 11.25).
The
concept can be explained using two theories:
1.
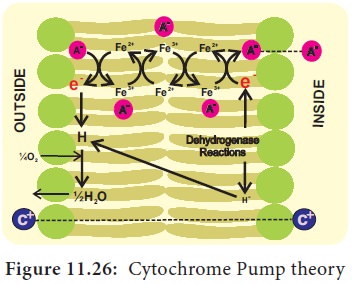
Lundegardh’s
Cytochrome Pump Theory:
Lundegardh and Burstrom (1933) observed
a correlation between respiration and anion absorption. When a plant is
transferred from water to a salt solution the rate of respiration increases
which is called as anion respiration
or salt respiration. Based on this observation Lundegardh (1950 and 1954) proposed cytochrome pump theory which is based on the following
assumptions:
i.  The
mechanism of anion and cation absorption are different.
ii. 
Anions are absorbed through cytochrome chain by an active process, cations are
absorbed passively.
iii.  An
oxygen gradient responsible for oxidation at the outer surface of the membrane and
reduction at the inner surface.
According
to this theory, the enzyme dehydrogenase on
inner surface is responsible for the
formation of protons (H+) and electrons (e–). As electrons pass outward through
electron transport chain there is a corresponding inward passage of anions.
Anions are picked up by oxidized cytochrome oxidase and are transferred to
other members of chain as they transfer the electron to the next component
(Figure 11.26).
The
theory assumes that cations (C+) move passively along the electrical gradient
created by the accumulation of anions (A–) at the inner surface of the
membrane.
Main
defects of the above theory are:
(i)
Cations also induce respiration.
(ii) Fails to
explain the selective uptake of ions.
(iii) It
explains absorption of anions only.
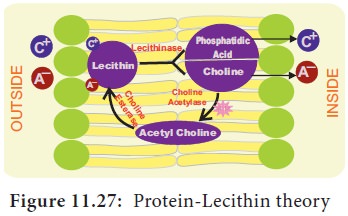
2.
Bennet-Clark’s
Protein-Lecithin Theory:
In 1956, Bennet -Clark proposed that the carrier
could be a protein associated with phosphatide
called as lecithin. The carrier is amphoteric (the ability to act either as an acid or a base) and
hence both cations and anions combine with it to form Lecithin-ion complex in the membrane. Inside the membrane, Lecithin-ion complex is broken down into phosphatidic acid and choline along with the liberation of
ions. Lecithin again gets regenerated from phosphatidic
acid and choline in the presence
of the enzyme choline acetylase and
choline esterase (Figure 11.27). ATP is required for regeneration of lecithin.
3. Donnan equilibrium
Within
the cell, some of the ions never diffuse out through the membrane. They are
trapped within the cell and are called fixed ions. But they must be balanced by
the ions of opposite charge. Assuming that a concentration of fixed anions is
present inside the membrane, more cations would be absorbed in addition to the
normal exchange to maintain the equilibrium. Therefore, the cation
concentration would be greater in the internal than in the external solution.
This electrical balance or equilibrium controlled by electrical as well as
diffusion phenomenon is known as the Donnan
equilibrium.
Related Topics